
��6�֣�(1)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol/L�������CH3COOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ ��
(2)�����ͬ��Ũ�Ⱦ�Ϊ0.2mo l/L�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ ��
l/L�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ ��
(3)�����ͬ��pH������13�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����9����m��n�Ĺ�ϵΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��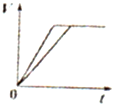 ��ʾ���ʵ���֮��Ϊ2��3��þ�����ֱ������ϡ���ᷴӦʱ����������������V����ʱ�䣨t���Ĺ�ϵ | B��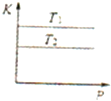 ��ʾ2SO2��g��+O2��g��?2SO3��g������H��0����ƽ�ⳣ��K��ѹǿP�Ĺ�ϵ��T1��T2 | C�� ��ʾ��0.1mol-L-1NaOH��Һ�ֱ�ζ�Ũ�Ⱦ�Ϊ0.1mol?L-1�����ͬ������ʹ��ᣬ���е������ǵζ���������� | D��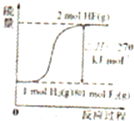 ��ʾ����ͬ������1 mol H2��g����1 mol F2��g����Ӧ����2 mol HF��g��ʱ���ų�����ͯΪ270kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�(1)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol/L�������CH3COOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ ��
(2)�����ͬ��Ũ�Ⱦ�Ϊ0.2mol/L�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ ��
(3)�����ͬ��pH������13�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����9����m��n�Ĺ�ϵΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�캣��ʡ�����и߶���ѧ�ڽ�ѧ������⻯ѧ�������Ծ� ���ͣ������
��6�֣�(1)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol/L�������CH3COOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ ��
(2)�����ͬ��Ũ�Ⱦ�Ϊ0.2mol/L�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ ��
(3)�����ͬ��pH������13�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����9����m��n�Ĺ�ϵΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com