
ĻÖÓŠŗ¬FeC12ŌÓÖŹµÄĀČ»ÆĶ¾§Ģå£ØCuC12?2H2O£©£¬ĪŖÖĘČ”“æ¾»µÄCuC12?2H2O£¬Ź×ĻČ½«ĘäÖĘ³ÉĖ®ČÜŅŗ£¬Č»ŗó°“ĻĀĶ¼ĖłŹ¾²½Öč½ųŠŠĢį“æ£ŗ
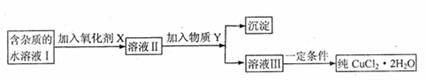
ŅŃÖŖH2O2”¢KMnO2”¢NaC1O”¢K2Cr2O7¾ł¾ßÓŠĒæŃõ»ÆŠŌ£¬ŅŖ½«ČÜŅŗÖŠµÄCu2+”¢Fe2+”¢Fe3+³ĮµķĪŖĒāŃõ»ÆĪļ£¬ŠčČÜŅŗµÄpH·Ö±šĪŖ6.4”¢6.4”¢3.7£¬
Ēė»Ų“šĻĀĮŠĪŹĢā
£Ø1£©±¾ŹµŃé×īŹŹŗĻµÄŃõ»Æ¼ĮXŹĒ ”£
A£®K2Cr2O7 B£®NaC1O C£®H2O2 D£®KMnO4
£Ø2£©ĪļÖŹY²»æÉÄÜŹĒ ”£
A£®CuO B£®CuC12 C£®Cu£ØOH£©2 D£®CuCO3
£Ø3£©³żČ„Fe3+µÄÓŠ¹ŲĄė×Ó·½³ĢŹ½ŹĒ£ØĪļÖŹYŌŚ£Ø2£©ÖŠŃ”ŌńŗĻŹŹµÄŃ”Ļī£©£ŗ ”£
£Ø4£©¼ÓČėŃõ»Æ¼ĮµÄÄæµÄŹĒ ”£
£Ø5£©×īŗóÄܲ»ÄÜÖ±½ÓÕō·¢½į¾§µĆµ½CuC12?2H2O£æ ”££ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©”£Čē²»ÄÜ£¬Ó¦ČēŗĪ²Ł×÷£æ£ØČēÄÜ£¬“ĖæÕ²»Ģī£© ”£
 Ó¦ÓĆĢā×÷Ņµ±¾ĻµĮŠ“š°ø
Ó¦ÓĆĢā×÷Ņµ±¾ĻµĮŠ“š°ø Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
Źī¼Ł×÷ŅµŹī¼ŁæģĄÖĮ·Ī÷°²³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com