
�����������ʵ���Ҫ���������ʽṹ,��ش��������⡣
(1)��֪A��BΪ��������Ԫ��,��ԭ�ӵĵ�һ�����ĵ��������±���ʾ:
| ������/kJ·mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1 817 | 2 745 | 11 578 |
| B | 738 | 1 451 | 7 733 | 10 540 |
Aͨ��������������,A�ĵ縺����������B�ĵ縺��(�>������<����=��)��
(2)�����Ĺ��������е�����ԼΪ399 kJ·mol-1�������±��йص����ʷ����� ��Ҫ��ѧ������Ϣ,˵�����峤ʱ������������Ƥ�������˺���ԭ��
��Ҫ��ѧ������Ϣ,˵�����峤ʱ������������Ƥ�������˺���ԭ��
����
��ɵ����ʵ���İ������е�̼ԭ���ӻ�����������������
| ���ۼ� | C—C | C—N | C—S |
| ����/kJ·mol-1 | 347 | 305 | 259 |
(3)ʵ��֤��:KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ����(��ͼ��ʾ),��֪3�����Ӿ���ľ������������±�:
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ·mol-1 | 786 | 715 | 3 401 |
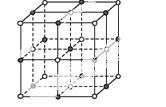
���4�����Ӿ���(������NaCl)�۵�Ӹߵ��͵�˳����:��������������MgO������һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+��������������
(4)���������Ӻ�δ�ɶԵ���Խ��,�����Խ��,�ż�¼����Խ�á�������������V2O5��CrO2��,�ʺ���¼�����ŷ�ԭ�ϵ�������������
(5)ij�����ķ��ӽṹ��ͼ��ʾ,������ڲ�����������(�����)��
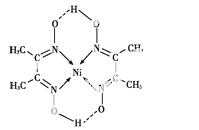
A.���Ӽ� B.���Լ�
C.������ D.���
E.��� F.�Ǽ��Լ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯ�ͺ�ú̿�ӹ������漰���ּ������豸��
(1)ʯ�ͷ���ʱ���ڲ�����____________(�ҵ�豸����)��Ͷ��ԭ�ϵ�ͬʱ��ò�Ʒ���ù���Ϊ____________�������̡�
(2)ʯ���ѻ���Ϊ���ѻ���________�ͼ����ѻ����ѻ���Ŀ�������________�IJ�����
(3)ú��ϴѡ��Ϊ�˽���ԭú�лҷֺ�________�ĺ�����ú��������ȼ����ָ�����ӵײ�����ú̿��������ʹȫ��ú̿����________����ȼ�յĹ��̡�
(4)ú��ֱ��Һ����ú���ʵ��ܼ���Ϻ��ڸ��º�________��������________________��������Һ��ȼ�ϵĹ��̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����
A���ϳɰ��ġ��������λ��������
B����Ƶ����Է�Һ�ü��кͺ�Ϳ����ŷ�
C����������Ĺ����У���Ϊ�������ϵ�����ú��������
D��ʹ��ú̿ת���Ĺܵ�ú����ֱ��ȼú�ɼ��ٻ�����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƶ�±����(NaX)���±����(SiX4)������������ȷ����
A��SiX4��ˮ��  B��SiX4�ǹ��ۻ�����
B��SiX4�ǹ��ۻ�����
C��NaX��ˮ�� D��NaX���۵�һ�����SiX4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ��H��C��N��O��F������Ҫ�ķǽ���Ԫ��,Fe��Cu��Ӧ�÷dz��㷺�Ľ�����
(1)FeԪ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ����������
(2)C��HԪ���γɵĻ���������й���16������,�÷����ЦҼ���м��ĸ�����Ϊ����������
(3)C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ(��Ԫ�ط��ű�ʾ)����������
(4)�ڲⶨHF����Է�������ʱ,ʵ����ֵһ���������ֵ,����Ҫԭ����
����
(5)C��N��Ԫ���γɵĻ�����C3N4�γɵ�ԭ�Ӿ���,�ṹ���ƽ��ʯ,����Ӳ�ȳ������ʯ,��ԭ����
����
(6)��ͼΪʯī�����ṹʾ��ͼ,�þ����к���Cԭ�ӵĸ���Ϊ����������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ֶ�����Ԫ�ص����ʡ���;��ԭ�ӽṹ��Ϣ���±���
| Ԫ�� | Ԫ�ص����ʡ���;��ԭ�ӽṹ��Ϣ |
| Q | ԭ�Ӻ�����6������ |
| R | �����������Ǵ�����������3�� |
| X | ��̬�⻯���ˮ��Һ���������ϣ��������� |
| Y | ��������Ԫ�صļ������������Ӱ뾶��С |
| Z | ����Ϊ����ɫ���壬�ڿ�����ȼ�շ�����ɫ���� |
����ݱ�����Ϣ�ش��������⣺
(1)Q�����̬�⻯����ӵĿռ乹��Ϊ________��
(2)R������X�������������·�Ӧ����Ϊ________������(����ۡ������ӡ�)���侧������Ϊ________��
(3)д��R��Z��ɽ������Ӽ��Ļ�����Ļ�ѧʽ��______________����ɵ���һ����������ѧ������Ϊ__________________��
(4)��ҵ���õ��Y��Z�γɻ������ˮ��Һ��ȡY���ʣ�д���÷�Ӧ�����ӷ���ʽ��_______________________��
(5)��1.01��105Pa��298 Kʱ��1.4 g QR������1.6 g R2��������ȫȼ�գ�����QR2����ʱ�ų�14.15 kJ������д��QRȼ�յ��Ȼ�ѧ����ʽ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����a mol FeBr2����Һ�У�ͨ��x mol Cl2�����и���ΪͨCl2������ ����Һ�ڷ�����Ӧ�����ӷ���ʽ����������ȷ����
����Һ�ڷ�����Ӧ�����ӷ���ʽ����������ȷ����
A��x��0.4a��2Fe2-+Cl2��2Fe3++2Cl-
B��x��0.6a��2Br��+ Cl2��Br2+2Cl��
C��x=a��2Fe2��+2Br��+2Cl2��Br2+2Fe3��+4Cl��
D��x=1.5a��2Fe2��+4Br��+3Cl2��2Br2+2Fe3��+6Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1) 2molO3��3 molO2������֮��___________��������֮��__________��ͬ��ͬѹ�µ��ܶ�֮��________������ԭ����֮��_______________�����֮��____________________��
(2) O3��Cl2�������Ƶ����ʣ�������������ˮ����������֪����������ʱ������ԭΪ��ͼ�̬������ͬ״����10LO3��____________L Cl2�����������൱��
(3)���廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��l 0 mL A���ȷֽ�����15 mLO2��10 mL F2����A�Ļ�ѧʽΪ____________�ƶϵ�����Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��������٤����������֪C2H4��C3H6�Ļ���������Ϊag����û����
A���������õ��Ӷ���ĿΪ��a/7+1��NA B������̼�����ĿΪaNA/7
C��ȼ��ʱ���ĵ�O2һ����33��6a/14L D������ԭ������ΪaNA/14
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com