


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
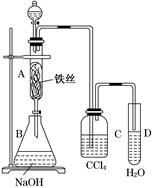
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������A��B��֧�ྻ�Թ��У����á� | |
| ����2����A�Թ��м��������Ba(OH)2��Һ�����ã����ˡ� | |
| ����3��ȡ��������2�õ��������������������ᡣ | |
| ����4��ȡ��������2�õ��ĵ���Һ������ �� | |
| ����5����B�Թ��м��� �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
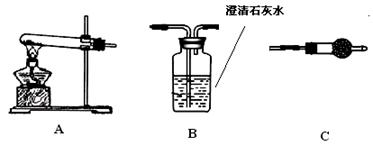
| | Bװ������Ʒ������(g) | Cװ�õ�����(g) | Dװ�õ�����(g) |
| ʵ��ǰ | 33.3 | 262.1 | 223.8 |
| ����� | 24 | 264.8 | 230.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
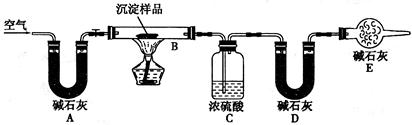
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
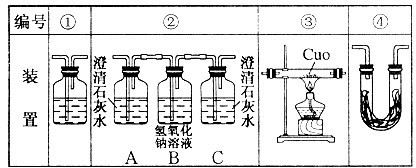
| A����ȡ�����֭������ˮϴ��δ��ϴ |
| B����ƿˮϴ��δ�ô���Һ��ϴ |
| C���ζ�ǰ���첿����һ���ݣ��ζ��յ�ʱ��ʧ |
| D���ζ�ǰ���Ӷ������ζ����Ӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
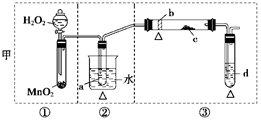
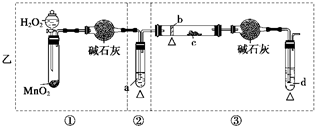
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com