
����Ŀ����1�������г�����һЩԭ�ӵ�2p�ܼ���3d�ܼ��е����Ų�����������ж���ЩΥ��������ԭ��________����ЩΥ���˺��ع���________��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��2��![]() ����������������ӽṹ��ͼ��ʾ��
����������������ӽṹ��ͼ��ʾ��

![]() ��������ԭ�ӵ��ӻ��������Ϊ________��
��������ԭ�ӵ��ӻ��������Ϊ________��
![]() ÿ��
ÿ��![]() �����к��еŵ��ӶԵ���ĿΪ________��
�����к��еŵ��ӶԵ���ĿΪ________��
��3����ѧ�Һϳ���һ����������![]() ������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2�������������˺��ֺϳ���һ�ֺ�����
������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2�������������˺��ֺϳ���һ�ֺ�����![]() ���Ļ�ѧʽΪ��
���Ļ�ѧʽΪ��![]() �������Ӿ��壬�����ʽΪ________������
�������Ӿ��壬�����ʽΪ________������![]() �м����֮��ļн�Ϊ
�м����֮��ļн�Ϊ![]() �����жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ________________��
�����жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ________________��
��4��ֱ�������������������������������������������ͨ�����ö�����ԭ�����������ģ���ͼ��ʾ������n�������������γɵ�������������ӵ�ͨʽΪ________��
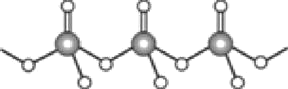
��5��̼�����е������Ӳ�ͬ���ȷֽ��¶ȾͲ�ͬ���±�Ϊ����̼���ε��ȷֽ��¶Ⱥͽ��������Ӱ뾶
̼���� |
|
|
|
|
�ȷֽ��¶� | 402 | 900 | 1172 | 1360 |
���������Ӱ뾶 | 66 | 99 | 112 | 135 |
���Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ����_____________��
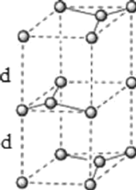
��6��ʯī�ľ���ṹ�;����ṹ��ͼ��ʾ����֪ʯī���ܶ�Ϊ![]() ��
��![]() ���ļ���Ϊ
���ļ���Ϊ![]() �������ӵ�������ֵΪ
�������ӵ�������ֵΪ![]() ����ʯī����IJ���Ϊ________cm��
����ʯī����IJ���Ϊ________cm��
 ��
��
���𰸡�![]()
![]()
![]()
![]()
![]()
![]()
![]() ̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ�
̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ� ![]()
��������
��1����������������ԭ�������ع�������ݷ����ش�
��2��![]() ��������ԭ�ӵŵ��Ӷ�����
��������ԭ�ӵŵ��Ӷ�����![]() ��
��![]() ������2��
������2��
![]() ��������ԭ�ӵŵ��Ӷ�����
��������ԭ�ӵŵ��Ӷ�����![]() ����ԭ�ӵŵ��Ӷ�����
����ԭ�ӵŵ��Ӷ�����![]() ��
��
��3��������������![]() ������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2���������������Ը�������һ����λ������ɣ�����ʽ��
������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2���������������Ը�������һ����λ������ɣ�����ʽ��![]() ��������
��������![]() ���Ļ�ѧʽΪ��
���Ļ�ѧʽΪ��![]() �������Ӿ��壬�����������
�������Ӿ��壬�����������![]() ��
��
��4����������ѧ���ɷ�������ͨʽ��
��5��̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣�
��6�������ĸߵ��ڲ����2����
![]() ����ԭ��ָ����ͬһ��ԭ�ӹ���ڲ���������������������ͬ�ĵ��ӣ����Υ������ԭ������
����ԭ��ָ����ͬһ��ԭ�ӹ���ڲ���������������������ͬ�ĵ��ӣ����Υ������ԭ������![]() �����ع���ָ���ǵ��Ӿ����ܷ�ռ��ͬ�����������������ͬ�����Υ�����ع������
�����ع���ָ���ǵ��Ӿ����ܷ�ռ��ͬ�����������������ͬ�����Υ�����ع������![]() ��
��
��2��![]() ��������ԭ�ӵŵ��Ӷ�����
��������ԭ�ӵŵ��Ӷ�����![]() ��
��![]() ������2���ӻ��������4��������ԭ�ӵ��ӻ��������Ϊ
������2���ӻ��������4��������ԭ�ӵ��ӻ��������Ϊ![]() ��
��
![]() ÿ����ԭ�ӵŵ��Ӷ���Ϊ1��ÿ����ԭ�ӵŵ��Ӷ�Ϊ2������ÿ��
ÿ����ԭ�ӵŵ��Ӷ���Ϊ1��ÿ����ԭ�ӵŵ��Ӷ�Ϊ2������ÿ��![]() �����к��еŵ��ӶԵ���ĿΪ10��
�����к��еŵ��ӶԵ���ĿΪ10��
![]() ������������
������������![]() ������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2���������������Ը�������һ����λ������ɣ������ӵĵ���ʽ��
������ṹ�ǶԳƵģ�5��N�ų���V���Σ�ÿ��N���ﵽ8�����ȶ��ṹ���Һ���2���������������Ը�������һ����λ������ɣ������ӵĵ���ʽ��![]() ��������
��������![]() ���Ļ�ѧʽΪ��
���Ļ�ѧʽΪ��![]() �������Ӿ��壬�����������
�������Ӿ��壬�����������![]() ����ѧʽΪ��
����ѧʽΪ��![]() �������Ӿ���ĵ���ʽΪ
�������Ӿ���ĵ���ʽΪ![]() ������
������![]() �м����֮��ļн�Ϊ
�м����֮��ļн�Ϊ![]() �����жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ
�����жԳ��ԣ�������ÿ��ԭ�ӵ�����������8�����ȶ��ṹ����ṹʽΪ![]() ��
��
![]() ���ݸ����Ľṹ������ѧ���ɷ���֪����n�������������γɵ�������������ӵ�ͨʽΪ
���ݸ����Ľṹ������ѧ���ɷ���֪����n�������������γɵ�������������ӵ�ͨʽΪ![]() ��
��
![]() ���Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ����̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ⣻
���Ž��������Ӱ뾶������̼���ε��ȷֽ��¶������ߣ�ԭ����̼���ηֽ�ʵ�ʹ����Ǿ����������ӽ��̼��������������ӣ�ʹ̼������ӷֽ�Ϊ������̼�Ĺ��̣����������������ͬʱ�������Ӱ뾶ԽС����������������Խǿ����Ӧ��̼���ξ�Խ���ֽ⣻
![]() ����ʯī�Ľṹ������֪ʯī�����к��е�̼ԭ����Ϊ
����ʯī�Ľṹ������֪ʯī�����к��е�̼ԭ����Ϊ![]() ����
����![]() ���ļ���Ϊrcm�����Ծ����ĵ����Ϊ
���ļ���Ϊrcm�����Ծ����ĵ����Ϊ![]() �����ݾ������ܶ�
�����ݾ������ܶ�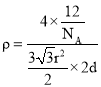 �����
�����![]() ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʱ����![]() ��Һ�еμ�
��Һ�еμ�![]() ��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ
��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ![]() ���������
���������![]() ������˵������ȷ����
������˵������ȷ����![]()
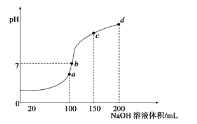
A.a��ʱˮ�ĵ���̶����
B.b��ʱ��Һ�е�����Ũ�ȹ�ϵ��![]()
C.c��ʱ��Һ�е�����Ũ�ȴ�С��ϵΪ![]()
D.d��ʱ��Һ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2.0molPCl3��1.0molCl2�������������ܱ������У���һ�������·���������Ӧ��PCl3(g)+Cl2(g)PCl5(g)��ƽ��ʱ��PCl5Ϊ0.4mol�������ʱ����1.0molPCl3��0.50molCl2������ͬ�¶����ٴ�ƽ��ʱPCl5�����ʵ����ǣ� ��
A. 0.4mol
B. 0.2mol
C. ��0.2mol
D. ����0.2mol����0.4mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��B��Mg�ڲ��Ͽ�ѧ�����й㷺��Ӧ�úͷ�չǰ����
�ش��������⣺
��1������Bԭ�ӹ������ʽ��ʾ��״̬�У��������ߵ���________![]() ����A������B��
����A������B��![]() ��
��
A. ![]() B.
B. ![]()
��2���������еĻ�����Ԫ��ͼ1��ʾ�����к���12��Bԭ�ӡ�![]() �õ�Ԫ�к���
�õ�Ԫ�к���![]() ������ĿΪ________��
������ĿΪ________��
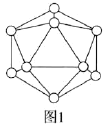
��3��![]() ����Ҫ�Ļ�ԭ�������������ӵ����幹��Ϊ________������ԭ�ӵ��ӻ���ʽΪ________��
����Ҫ�Ļ�ԭ�������������ӵ����幹��Ϊ________������ԭ�ӵ��ӻ���ʽΪ________��
��4����̬Mgԭ�ӵĺ�������Ų�ʽΪ________��������������Ԫ���У��縺��С��MgԪ�ص���________![]() ��Ԫ�ط��ţ���ͬ
��Ԫ�ط��ţ���ͬ![]() ��ԭ�ӵ�һ������С��Mgԭ�ӵ���________��
��ԭ�ӵ�һ������С��Mgԭ�ӵ���________��
��5��![]() ��
��![]() ������Ϊ�ͻ���ϣ���ԭ����________��
������Ϊ�ͻ���ϣ���ԭ����________��
��6��![]() �����ڹ�ѧ�������䳤�����;����ṹ��ͼ2��ʾ��
�����ڹ�ѧ�������䳤�����;����ṹ��ͼ2��ʾ��
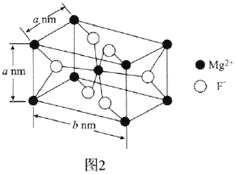
![]() ����λ��Ϊ________��
����λ��Ϊ________��
![]() �������ӵ�������ֵΪ
�������ӵ�������ֵΪ![]() ����
����![]() ������ܶȿɱ�ʾΪ________
������ܶȿɱ�ʾΪ________![]() �ú�a��b��
�ú�a��b��![]() �Ĵ���ʽ��ʾ
�Ĵ���ʽ��ʾ![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������������������ɵ���Ⱦ��Ŀǰ�о�����Ҫ���⡣
(1)��ҵ�ϳ��û���̿��ԭһ���������䷴ӦΪ��2NO(g)+C(s) N2(g)+CO2(g)�����ݻ���Ϊl L�ļס��ҡ����������ݺ��������зֱ���������Ļ���̿��һ������NO����ø�������n(NO)�淴Ӧʱ��t�ı仯������±���ʾ��
0min | 40min | 80min | 120min | 160min | ||
�� | T�� | 2mol | 1.45 mol | 1 mol | 1 mol | 1 mol |
�� | 400�� | 2 mol | 1.5 mol | 1.1 mol | 0.8 mol | 0.8 mol |
�� | 400�� | 1 mol | 0.8 mol | 0.65 mol | 0.53 mol | 0.45 mol |
��������Ӧ�¶�T��______400��(����>����<������=��)���������У�0~40min��ƽ����Ӧ����v(CO2)=_____________________���������д�ƽ���NO�����ʵ���Ϊ_________mol��
(2)����̿��ԭNO2�ķ�ӦΪ��2NO2(g)+2C(s) N2(g)+2CO2(g)���ں��������£�l mol NO2����������̿�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ��ͼ��ʾ��
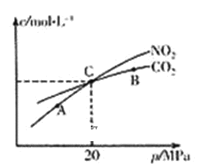
��A��B��C������NO2��ת������ߵ���_________��(����A������B������C��)��
�ڼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��KP=_______MPa(Kp����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
(3)ȼú�������������·�����
�����٣����������Ƚ���(��Ҫ�ɷ�CO��CH4��H2)��SO2�ڸ����»�ԭ�ɵ������漰�IJ��ַ�Ӧ���£�
2CO(g)+SO2(g)=S(g)+2CO2(g) ��H1=8.0 kJ��mol-1
2CO(g)+O2(g)=2CO2(g) ��H2=��566.0kJ��mol-1
2H2(g)+O2(g)=2H2O(g) ��H3=��483.6 1kJ��mol-1
��H2(g)��ԭSO2(g)����S(g)��H2O(g)���Ȼ�ѧ����ʽΪ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���![]() ��Һ����μ���
��Һ����μ���![]() ���ᣬ��Һ��R�������ʵ���������pH��ϵ��ͼ��ʾ
���ᣬ��Һ��R�������ʵ���������pH��ϵ��ͼ��ʾ![]() ���ȶ�����ת��Ϊ
���ȶ�����ת��Ϊ![]() �����ݳ���Һ�������ݳ�δ�����������������ݳ��������Һ����仯
�����ݳ���Һ�������ݳ�δ�����������������ݳ��������Һ����仯![]() ����˵���������
����˵���������![]()
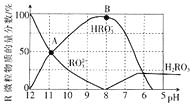
A.![]() ��Һ�У�
��Һ�У�![]()
B.����Һ![]() ʱ����Һ���������40mL
ʱ����Һ���������40mL
C.��B���Ӧ����Һ�У�����Ũ��������![]()
D.A���ӦpHԼΪ![]() ��
��![]() ��ˮ�ⳣ��
��ˮ�ⳣ��![]() ������Ϊ
������Ϊ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������һ��ʵ��װ������ⱥ��ʳ��ˮ�������������������������(Լ6 mL)�ͼ���������������(��Ӧ��������������������)��
(1)�Դ���ͼ��ѡ�ü��ֱ�Ҫ������������һ����װ�ã����������ӿڵ�����˳��(����)��A��G-F-I��B��__________��
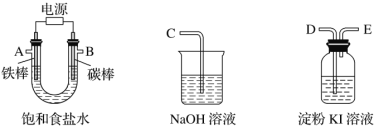
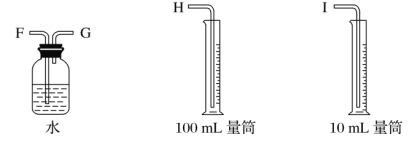
(2)������ֱ����Դ��________����̼���Ϸ����ĵ缫��ӦΪ_____________________________________��
(3)��˵���������������Ե�ʵ��������_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶�����һ��������ܱ������У���������������Ϊ���淴ӦA(g)��3B(g) ![]() 2C(g)�ﵽƽ��״̬��־���� ( )
2C(g)�ﵽƽ��״̬��־���� ( )
��C������������C�������������
����λʱ��������a mol A��ͬʱ����3a mol B
��A��B��C��Ũ�Ȳ��ٱ仯
��C�����ʵ������ٱ仯
������������ѹǿ���ٱ仯
���������������ʵ������ٱ仯
����λʱ������a mol A��ͬʱ����3a mol B
��A��B��C�ķ�����֮��Ϊ1��3��2
A. �ڢ�B. �ܢ�C. �٢�D. �ݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��2RCH2CHO![]() RCH2CH=CRCHO��H2O��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
RCH2CH=CRCHO��H2O��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�

��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ_________���ṹ������ʾAֻ��һ������A������Ϊ___________��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________��
��3��C��____�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���______________________________________________________��
��4�������ķ�Ӧ����Ϊ_________________��D���������ŵ�����Ϊ________________��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��_________________________��
a�������к���6��̼ԭ����һ������ b�����������������Ű���ˮ������еĹ�����
��6���������ķ�Ӧ����Ϊ___________��д��E�Ľṹ��ʽ_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com