
�ۻ�ѧһһ��ѧ�뼼���ݣ�15�֣�
�ȼҵ��������Ļ�ѧ��ҵ֮һ������Ĥ��ⷨΪĿǰ�ձ�ʹ�õ�������������������������ͼ��ʾ��
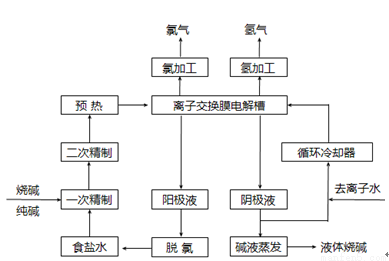
��1���������п���ѭ���������� ��
��2����ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к���Ca2+��Mg2+��SO42���������ʣ������ڽ������ǰ��Ҫ�������ξ��ƣ�д��һ�ξ����з��������ӷ���ʽ ����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۻ����ʲô��� ��
��3��ͼ�ǹ�ҵ�ϵ�ⱥ��ʳ��ˮ�����ӽ���Ĥ����ʾ��ͼ(�����ý��������Ƴɣ�������̼�����Ƴ�)����B�������������� ��E�缫�������� ������ܷ�Ӧ�����ӷ���ʽΪ ��
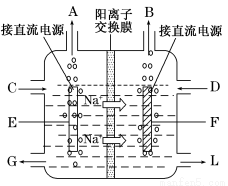
��4���������۳����ĵ���ˮ�У����������������ܽ��ȣ���Ҫ����8����9��������������Һ���䳹�׳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����֪�ڵ����У�ÿСʱͨ��1�����ֱ������Բ���1.492g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1.342��/m3��113m3�����۵ĵ���ǿ��1.45 ��104A���õ��۵ĵ��Ч��Ϊ ��
��1���Ȼ��� �������ƣ�2�֣�
��2��Ca2++ CO32�� = CaCO3�� Mg2+ + 2OH�� = Mg(OH)2�� ��2�֣����Լ����������ˮ�л���������Mg2+��Ca2+�����������»����ɳ����������ӽ���Ĥ��2�֣���
��3��H2 ���� 2Cl����2H2O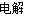 Cl2����H2����2 OH����4�֣�
Cl2����H2����2 OH����4�֣�
��4��Na2SO3 + Cl2 + H2O = Na2SO4 + 2HCl��2�֣�
��5��93.46%��3�֣�
��������
��������� (1)�������������ͼ֪��δ����NaCl�Ͳ���NaOH����ѭ��ʹ�á���2������ˮ�к���Ca2+��Mg2+��SO42���������ʣ�һ�ξ���ʱ���봿����ռ����CO32-ʹCa2+������OH��ʹMg2+���������ӷ���ʽΪCa2++ CO32�� = CaCO3�� Mg2+ + 2OH�� = Mg(OH)2�������Լ����������ˮ�л���������Mg2+��Ca2+����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۣ����������»����ɳ����������ӽ���Ĥ����3��ͼ��Na+�����ͨ�����ӽ���Ĥ�����Ҳ࣬˵��F�缫Ϊ������B��������������H2��E�缫������������ܷ�Ӧ�����ӷ���ʽ2Cl����2H2O Cl2����H2����2 OH������4���ܽ���������������Һ��Ӧ���������ƺ����ᣬ��ѧ����ʽΪNa2SO3 + Cl2 + H2O = Na2SO4 + 2HCl����5��
Cl2����H2����2 OH������4���ܽ���������������Һ��Ӧ���������ƺ����ᣬ��ѧ����ʽΪNa2SO3 + Cl2 + H2O = Na2SO4 + 2HCl����5��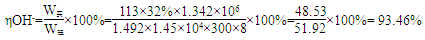
���㣺�ȼҵ������ת�����̺͵��ԭ����Ӧ�á�


 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡ�����и�����ѧ�������Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�������ȫ��ȷ����
��� | ʵ�� | ���� |
A | ����ˮ��Ӧ | �����Ӵ�ú����ȡ�������ƣ������̶���С���ƣ�С�ķ���װ��ˮ���ձ��� |
B | ����һ��Ũ�ȵ��Ȼ�����Һ1000mL | ȷ��ȡ�Ȼ��ع��壬���뵽1000mL������ƿ�У���ˮ�ܽ⣬��ҡ�ȣ����� |
C | �ų���ʽ�ζ��ܼ��첿�ֵ����� | ����������ʹ��������б���ϣ�����ָ��ס���ܣ����ἷѹ�����飬ʹ��Һ�Ӽ������� |
D | ȡ����Һ©����������ϲ�Һ�� | �²�Һ��ӷ�Һ©���¶˹ܿڷų����رջ�������һ�������������ϲ�Һ������ӷ�Һ©���¶˹ܿڷų� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡ��ˮ�и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���������в����ںϽ���ǣ�������
A��Ӳ�� B������ C����ͭ D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡʯ��ׯ����У����������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�����������Һ���ܴ����������
A�� ��
�� ��
�� ��
��
B�� ��
�� ��
�� ��
��
C�� ��
�� ��
�� ��
��
D�� ��
�� ��
�� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡʯ��ׯ����У����������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и������ӷ�Ӧ����H����OH�� H2O��ʾ����
H2O��ʾ����
A���������������� B����������������
C����������������� D���������ƺ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡ��ɽ�и���10���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��pAg=-lg{c(Ag+)},KspAgCl=1��10-12��ͼ����10mLAgNO3��Һ������0.1 mol/L��NaCl��Һʱ����Һ��pAg���ż���NaCl��Һ���������λmL���仯��ͼ��(ʵ��)������ͼ���������н�����ȷ���ǣ� ��
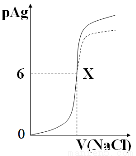
����ʾ��KspAgCl��KspAgI��
A��ԭAgNO3��Һ�����ʵ���Ũ��Ϊ0.1 mol��L-1
B��ͼ��x�������Ϊ��10��6 ��
C��ͼ��x���ʾ��Һ��Ag+ ��Cl�� Ũ����ͬ
D����0.1 mol��L-1��NaCl����0.1 mol��L-1NaI��ͼ�����յ���Ϊ���߲���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ��ɫ��У������һ��������ѧ�Ծ��������棩 ���ͣ������
��11�֣� (��) �����£���30ml�� Al2(SO4)3��Һ�У���μ���1.0 mol��L��1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����ı仯������ͼ��ʾ��

��1��д��a����Һ�����Ե����ӷ���ʽ��
��2����c��ʱV��NaOH��Ϊ90ml����Al2(SO4)3��Һ�����ʵ���Ũ��ԼΪ��
��3��д��b��c�η�Ӧ�����ӷ���ʽ�� ��
��4��d��ʱ��V��NaOH��ԼΪ
(��)��������Ũ�Ⱦ�Ϊ0.5 mol/L��������Һ��
��Na2CO3��Һ����NaHCO3��Һ����HCl��Һ���ܰ�ˮ
��1������м��������Ȼ�粒��壬��ʱc(NH4+)/c(OH��)��ֵ________(���������С�����䡱)��
��2�������ۺܵ͢���Һ��Ϻ���Һǡ�ó���������ǰ�۵����________�ܵ����(����ڡ�����С�ڡ����ڡ�)����ʱ��Һ������Ũ���ɴ�С��˳����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ��ɫ��У������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йػ�ѧ������ȷ���� ( )
A��������Ľṹʽ��H��Cl��O B��������̼�ı���ģ�ͣ� 
C�����ĵ���ʽ�� D����ά�ص�ͨʽ��(C6H12O6)n
D����ά�ص�ͨʽ��(C6H12O6)n
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�����ڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�±�������������������ȷ���һ�Ϊ�����ϵ����
ѡ�� | ������ | ������ |
A | NH4Cl��ǿ�������� | ��NH4Cl��Һ���ɿ��Ʊ�NH4Cl���� |
B | Fe3+��ǿ������ | ��KSCN��Һ���Լ���Fe3+ |
C | SO2�л�ԭ�� | ��SO2��ʹ��ˮ��ɫ |
D | Cl2��Ư���� | ��Cl2����ʯ���鷴Ӧ�Ʊ����������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com