
| A�����������Һ�¶����ߣ�HF�ĵ���̶ȼ�С�������ǻӷ��� |
| B��ˮ������Ȼ�ѧ����ʽΪ��H2O��1��?H+��aq��+OH-��aq����H=+57.3kJ/mol |
| C����c��0.1ʱ��һ�������ڣ�c��Na+��=c��F-�� |
| D������Ϻ���Һ�У�c��Na+����c��OH-����c��F-����c��H+������cһ��С��0.1 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ�м���ϡ��ˮ��ƽ�������ƶ���c��OH-������ |
| B����ˮ�м������������������ƣ�c��H+������Kw��� |
| C����ˮ�м����Ȼ�����Һ��c��H+�����䣬n��H+����� |
| D����ˮ���ȣ�Kw����pH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�� ��� | HA�����ʵ���Ũ�ȣ�mol?L-1�� | NaOH�����ʵ���Ũ�ȣ�mol?L-1�� | ��Ϻ���Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
| CH3COOH | H2CO3 | H2S |
| 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | K1=9.1��10-8 K2=1.1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������Ǿۺ��� |
| B��CH2=CH-CH3�Ǿ���ϩ�ĵ��� |
| C�����ϵ���Ҫ�ɷ��Ǻϳ���֬ |
| D�����Ͽ��Է�Ϊ�ȹ��Ժ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
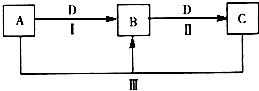 A��B��C��D������ѧ��ѧ���������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ֲ�������ȥ����
A��B��C��D������ѧ��ѧ���������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ֲ�������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ݻ���Ϊ500 mL�Ģ������ܱ���������������㶨���䣩�зֱ����1 mol N2��2.5 mol H2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䣬������������ͬ������·�����Ӧ��N2+3H2?2NH3����H��0����ʵ���÷�Ӧ�����е�t minʱN2�����������ͼ��ʾ������˵����ȷ���ǣ�������
�ݻ���Ϊ500 mL�Ģ������ܱ���������������㶨���䣩�зֱ����1 mol N2��2.5 mol H2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䣬������������ͬ������·�����Ӧ��N2+3H2?2NH3����H��0����ʵ���÷�Ӧ�����е�t minʱN2�����������ͼ��ʾ������˵����ȷ���ǣ�������| A����v��H2��=3v��N2��ʱ������˵�����������еķ�Ӧ��ƽ��״̬ |
| B����t minʱ��һ���ﻯѧƽ��״̬���Ǣ�͢� |
| C����t minʱ���c��N2����=1 mol/L����������г���1.5 mol N2��1 mol NH3��H2��ת���ʲ��� |
| D�����������еķ�Ӧ���ﵽƽ�������I�л�������ƽ����Է���������С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com