
ĻĀĮŠÖŠŃ§»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼ÖŠ£¬·“Ó¦Ģõ¼ž¼°²æ·Ö·“Ó¦ĪļŗĶ²śĪļĪ“Č«²æ×¢Ć÷£¬ŅŃÖŖA£®DĪŖ½šŹōµ„ÖŹ£¬ĘäĖū¾łĪŖ»ÆŗĻĪļ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
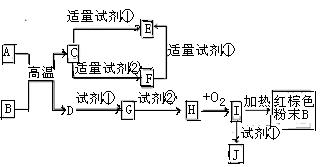
Š“³öĪļÖŹCµÄ»ÆѧŹ½£ŗ
Š“³öĪļÖŹBµÄŅ»ÖÖÓĆĶ¾£ŗ
£Ø3£©ŅŌJµÄ±„ŗĶČÜŅŗÖʱø½ŗĢåµÄ²Ł×÷ĪŖ
ӣ
Š“³öĻĀĮŠ·“Ó¦µÄ·½³ĢŹ½£ŗ
A”śFµÄĄė×Ó·½³ĢŹ½ £®
H”śIµÄ»Æѧ·½³ĢŹ½
ŹŌ¼Į¢ŚæÉŅŌÓÉŅ»ÖÖµ»ĘÉ«¹ĢĢå·ŪÄ©ŗĶŅ»ÖÖ³£¼ūŅŗĢå·¢Éś·“Ó¦¶ųÖĘµĆ£¬ĘäĻąÓ¦µÄ»Æѧ·½³ĢŹ½
£Ø5£©Ä³Ķ¬Ń§ČĻĪŖJÖŠæÉÄÜĶ¬Ź±ŗ¬ÓŠĶ¬Ņ»ÖÖ½šŹōŌŖĖŲµÄĮ½ÖÖŃōĄė×Ó£¬ČōŅŖČ·ČĻĘäÖŠµĶ¼Ū½šŹōŃōĄė×ӵēęŌŚ£¬Ó¦Ń”ÓĆ £ØŃ”ĢīŠņŗÅ£©
A”¢KSCNČÜŅŗŗĶĀČĖ® B”¢Ģś·ŪŗĶKSCNČÜŅŗ C”¢ÅØ°±Ė® D”¢ĖįŠŌøßĆĢĖį¼Ų
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĻĀĮŠÖŠŃ§»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼ÖŠ£¬·“Ó¦Ģõ¼ž¼°²æ·Ö·“Ó¦ĪļŗĶ²śĪļĪ“Č«²æ×¢Ć÷£¬ŅŃÖŖA”¢DĪŖ½šŹōµ„ÖŹ£¬ĘäĖūĪŖ»ÆŗĻĪļ£®
ĻĀĮŠÖŠŃ§»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼ÖŠ£¬·“Ó¦Ģõ¼ž¼°²æ·Ö·“Ó¦ĪļŗĶ²śĪļĪ“Č«²æ×¢Ć÷£¬ŅŃÖŖA”¢DĪŖ½šŹōµ„ÖŹ£¬ĘäĖūĪŖ»ÆŗĻĪļ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÖŠŃ§»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼ÖŠ£¬·“Ó¦Ģõ¼ž¼°²æ·Ö·“Ó¦ĪļŗĶ²śĪļĪ“Č«²æ×¢Ć÷£¬ŅŃÖŖA£®DĪŖ½šŹōµ„ÖŹ£¬ĘäĖū¾łĪŖ»ÆŗĻĪļ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

Š“³öĪļÖŹCµÄ»ÆѧŹ½£ŗ
Š“³öĪļÖŹBµÄŅ»ÖÖÓĆĶ¾£ŗ
£Ø3£©ŅŌJµÄ±„ŗĶČÜŅŗÖʱø½ŗĢåµÄ²Ł×÷ĪŖ
ӣ
Š“³öĻĀĮŠ·“Ó¦µÄ·½³ĢŹ½£ŗ
A”śFµÄĄė×Ó·½³ĢŹ½ £®
H”śIµÄ»Æѧ·½³ĢŹ½
ŹŌ¼Į¢ŚæÉŅŌÓÉŅ»ÖÖµ»ĘÉ«¹ĢĢå·ŪÄ©ŗĶŅ»ÖÖ³£¼ūŅŗĢå·¢Éś·“Ó¦¶ųÖĘµĆ£¬ĘäĻąÓ¦µÄ»Æѧ·½³ĢŹ½
£Ø5£©Ä³Ķ¬Ń§ČĻĪŖJÖŠæÉÄÜĶ¬Ź±ŗ¬ÓŠĶ¬Ņ»ÖÖ½šŹōŌŖĖŲµÄĮ½ÖÖŃōĄė×Ó£¬ČōŅŖČ·ČĻĘäÖŠµĶ¼Ū½šŹōŃōĄė×ӵēęŌŚ£¬Ó¦Ń”ÓĆ £ØŃ”ĢīŠņŗÅ£©
A”¢KSCNČÜŅŗŗĶĀČĖ® B”¢Ģś·ŪŗĶKSCNČÜŅŗ C”¢ÅØ°±Ė® D”¢ĖįŠŌøßĆĢĖį¼Ų
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģÕć½Ź”øßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ĻĀĮŠÖŠŃ§»Æѧ֊³£¼ūĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼ÖŠ£¬·“Ó¦Ģõ¼ž¼°²æ·Ö·“Ó¦ĪļŗĶ²śĪļĪ“Č«²æ×¢Ć÷£¬ŅŃÖŖA”¢DĪŖ½šŹōµ„ÖŹ£¬ĘäĖūĪŖ»ÆŗĻĪļ£®

ŹŌĶʶĻ£ŗ
(1)Š“³öĪļÖŹµÄ»ÆѧŹ½£ŗA£ŗ____________”¢C£ŗ____________”¢I£ŗ____________.
(2)Š“³öĻĀĮŠ·“Ó¦µÄ·½³ĢŹ½£ŗ
C”śFµÄĄė×Ó·½³ĢŹ½______________________________________________£®
H”śIµÄ»Æѧ·½³ĢŹ½________________________________________________£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com