
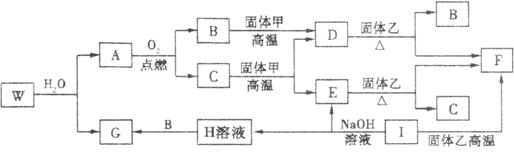
�Իش��������⣺
(1)A�ķ���ʽ_________�������ҵĻ�ѧʽ������_________��
(2)����ת���У���C![]() E����D
E����D![]() F����E
F����E![]() F����I
F����I![]() F����A
F����A![]() B���������û���Ӧ����(�����)_________��
B���������û���Ӧ����(�����)_________��
(3)д��W��ˮ��Ӧ�Ļ�ѧ����ʽ___________________________��
I��NaOH��Һ��Ӧ�����ӷ���ʽ___________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ͼ�ѧ�����ר��ѧУ�߶����ڽ�ҵ�������ƻ�ѧ�Ծ����������� ���ͣ������
��֪����ס�E��I��F��Ϊ�����ĵ��ʣ�����E�ڳ�����Ϊ��̬��GΪ��ɫ��״�����������ܽ������ᣬ�����ܽ�������������Һ��A�ڳ�����Ϊ��̬����������ȫ��Ӧʱ���������1:2��W�������ִ��ڲ�ͬ�����ڵ�Ԫ����ɵĻ������ˮ��Ӧ����A��Gʱ�Ļ�ѧ������֮��Ϊ1:3:3:1��������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�IJ���δȫ����ʾ�����Իش��������⣺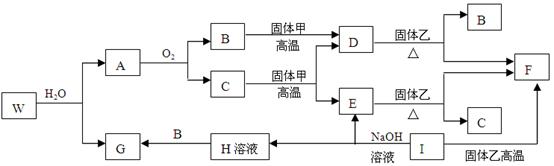
��1��B�ĵ���ʽΪ �������ҵĻ�ѧʽ������ ��
��2������ת���У���C��E ��D��F ��E��F ��I��F ��I��E ��A��B ������
�û���Ӧ���ǣ�����ţ� ��
��3��д��W��ˮ��Ӧ�Ļ�ѧ����ʽ_____________________________________________,
I��NaOH��Һ��Ӧ�����ӷ���ʽ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ͼ�ѧ�����ר��ѧУ�߶����ڽ�ҵ�������ƻ�ѧ�Ծ��������棩 ���ͣ������
��֪����ס�E��I��F��Ϊ�����ĵ��ʣ�����E�ڳ�����Ϊ��̬��GΪ��ɫ��״�����������ܽ������ᣬ�����ܽ�������������Һ��A�ڳ�����Ϊ��̬����������ȫ��Ӧʱ���������1:2��W�������ִ��ڲ�ͬ�����ڵ�Ԫ����ɵĻ������ˮ��Ӧ����A��Gʱ�Ļ�ѧ������֮��Ϊ1:3:3:1��������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�IJ���δȫ����ʾ�����Իش��������⣺

��1��B�ĵ���ʽΪ �������ҵĻ�ѧʽ������ ��
��2������ת���У���C��E ��D��F ��E��F ��I��F ��I��E ��A��B ������
�û���Ӧ���ǣ�����ţ� ��
��3��д��W��ˮ��Ӧ�Ļ�ѧ����ʽ_____________________________________________,
I��NaOH��Һ��Ӧ�����ӷ���ʽ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
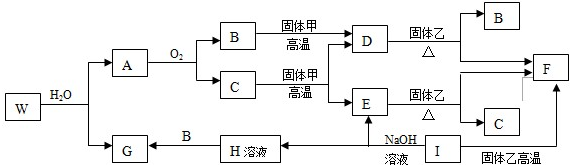
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com