
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

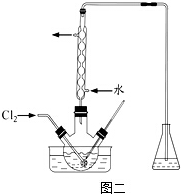
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
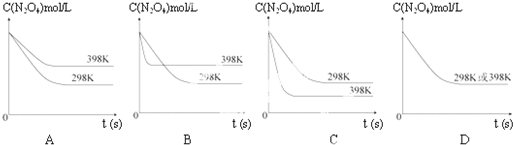
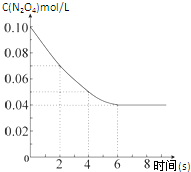 ���¶�Ϊ298Kʱ����0.10mol��ɫ��N2O4�������1L��յ��ܱ������У����ֺ���ɫ��ֱ������N2O4��g��?2NO2��g����ƽ�⣮��ͼ��ʾ�ⶨN2O4��Ũ����ʱ���ϵ�����ߣ�������ΪN2O4��Ũ�ȣ�������Ϊʱ�䣩��
���¶�Ϊ298Kʱ����0.10mol��ɫ��N2O4�������1L��յ��ܱ������У����ֺ���ɫ��ֱ������N2O4��g��?2NO2��g����ƽ�⣮��ͼ��ʾ�ⶨN2O4��Ũ����ʱ���ϵ�����ߣ�������ΪN2O4��Ũ�ȣ�������Ϊʱ�䣩��| T/K | 310 | 320 |
| Kֵ | 0.38 | 0.42 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��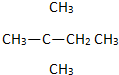 |
| B��CH3 CH2 CH2 CH2 CH3 |
C��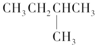 |
D��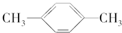 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ȶ��ԣ�CH4��SiH4 |
| B�����ԣ�H2SO4��HClO4 |
| C���۵㡢�е㣺O2��S |
| D��ԭ�Ӱ뾶��N��P |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ʯ���������ڵ��� |
| B��Ư�ۡ�ʯӢ�����ڴ����� |
| C���Ȼ�李������ᶼ����ǿ����� |
| D�������ǡ������ʶ����ڸ߷��ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
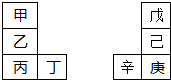 �ס�����Ԫ�������ڱ��е����λ����ͼ���������ԭ���������3�����һ�ֵ�������Ȼ��Ӳ���������ʣ���������ͬ����Ԫ�أ������ж���ȷ���ǣ�������
�ס�����Ԫ�������ڱ��е����λ����ͼ���������ԭ���������3�����һ�ֵ�������Ȼ��Ӳ���������ʣ���������ͬ����Ԫ�أ������ж���ȷ���ǣ�������| A�������ԣ��ף��ң��� |
| B��ԭ�Ӱ뾶������������ |
| C���������ԭ�Ӻ�����������13 |
| D���ҵĵ����ڿ�����ȼ������ֻ�����Ӽ��Ļ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com