
ij��ѧС������ͼװ�õ��CuCl2��Һ������Ư��Һ��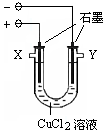

��1���������ķ�Ӧʽ�ǣ� ��������W��Ӧ������� ���ӡ�
��2��ʵ���������̼���ϳ��˸����к�ɫ���ʣ���������������ɫ���ʡ�
����������ʾ��
| �������Ƽ���ѧʽ | �Ȼ���ͭCuCl | ��ʽ�Ȼ�ͭCu2( OH)3Cl |
| ���� | ��ɫ���塢����ˮ | ��ɫ���塢����ˮ |

��1��2Cl- - 2e- = Cl2���� X ��2��Cu
��3���� ͭ�������ɫ����������Cu2O��
��Ag++Cl- = AgCl��
��2CuCl+H2 2Cu+2HCl
2Cu+2HCl
��Cu2+ + e- +Cl- = CuCl��
�ݣ�W1-W2��/35.5 mol��ƫС
���������������1���ö��Ե缫���CuCl2��Һʱ������ͬ�ֵ����ų⣬���ֵ���������ԭ���������������ŵ硣���������ӵķŵ�����Cl->OH-.�������ķ�Ӧʽ�ǣ�2Cl- - 2e- = Cl2����XΪ����YΪ������������W��Ӧ�������X���ӣ�������ӦCl2��2NaOH=NaCl��NaClO��H2O������ȡ������Һ��(2)�ٺ�ɫ���ʿ�����Cu; ��3����ʵ���У�̼���ϵİ�ɫ������ȫ��Ϊ��ɫ����ˮ����ͭ����ɫ��֤���ں�ɫ�����в���OԪ�أ�Ҳ�Ͳ���Cu2O��d�г��ְ�ɫ����֤��������AgCl��������ʱ̼���ϵĺ�ɫ������ͭ, ��ˮ����ͭ�������Ǽ����ɫ����������Cu2O����d�з�Ӧ�����ӷ���ʽ��Ag++Cl- = AgCl��������װ��b�з�����Ӧ�Ļ�ѧ����ʽ��2CuCl+H2 2Cu+2HCl���� ���CuCl2��Һʱ�������ϲ�����ɫ���ʵ�ԭ����Cu2+ + e- +Cl- = CuCl�����ݸ���Ԫ���غ��֪�������ϲ�����ɫ���ʵ����ʵ���Ӧ�õ���Cu�����ʵ����������ʵ����ǣ�W1-W2��/35.5 mol����װ��b��ȴʱ������ͨH2����һ����Cu�ͻᱻ�����е�����������ʹW2ƫ�����գ�W1-W2��/35.5��������Cu+�IJ��ʻ�ƫС��
2Cu+2HCl���� ���CuCl2��Һʱ�������ϲ�����ɫ���ʵ�ԭ����Cu2+ + e- +Cl- = CuCl�����ݸ���Ԫ���غ��֪�������ϲ�����ɫ���ʵ����ʵ���Ӧ�õ���Cu�����ʵ����������ʵ����ǣ�W1-W2��/35.5 mol����װ��b��ȴʱ������ͨH2����һ����Cu�ͻᱻ�����е�����������ʹW2ƫ�����գ�W1-W2��/35.5��������Cu+�IJ��ʻ�ƫС��
���㣺������ԭ����Ӧ�á��缫ʽ����д�����ʵijɷֵ�ȷ����ij���ʺ����IJⶨ����������֪ʶ��


 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�������������Ҫ�Ĺ�ҵ��Ʒ����ش�
��1����������������Һ��Ӧ�����ӷ���ʽ�� ��
��2����ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ��
�ٸõ��۵�������Ӧʽ�� ��
��ͨ�翪ʼ������������ҺpH�����������ԭ�� ��
�۳�ȥ���ʺ������������Һ��Һ����� (��д��A����B��)������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�����ͼװ����ʾ����������ȼ�ϵ��B���е�ij���ʵ�飺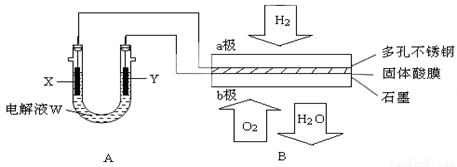
(1)�����Bʹ�����ǰ����(Li2NH)������Ϊ������ϣ��䴢��ԭ���ǣ�Li2NH��H2��LiNH2��LiH��������˵������ȷ����________��
| A��Li2NH��N�Ļ��ϼ���-1�� | B���÷�Ӧ��H2�������������ǻ�ԭ�� |
| C��Li�� ��H�������Ӱ뾶��� | D���˷������ƿ�����ԭ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(11��)��֪��������Խ���γ�ԭ���ʱԽ���ŵ硣�������ͼװ�ã��ش��������⣺
��1����װ�������ǣ�A��________��B��______��C��________��
��2��д���缫�Ϸ����ķ�Ӧ����_____________����___________����_____________��
��3������·����2 mol��������ʱ���ټ��������仯______g�����������仯______g��
��4����Ӧ����һ��ʱ���A��B��C�����е������ҺŨ�Ȳ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(27��)��1����������������Ӧ����NaOH + HCl =" NaCl" + H2O;��Fe +H2SO4 = FeSO4 + H2 ��
����Ӧ��Ϊ���ȷ�Ӧ���� ������Ƴ�ԭ��ص��� ��������ţ�
��2��������ͼ��բٵ��Ӵ� Ƭ��������Һ��H+�� Ƭ�ƶ���
�� ������������ ������ ��Ӧ����д����������ԭ������
�۸����ĵ缫����ʽΪ
�� ����Ӧ��������0.01mol���ӷ���ת�ƣ������ɵ������ڱ�״���µ����Ϊ L��
��3��д������Ȼ�ͭ��Һʱ�������ĵ缫��Ӧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Ӧ�з�Ӧ�����������У�FeCl2��FeCl3��CuCl2��Cu��
��1����������Ӧ��Ƴɵ�ԭ�����ͼ����ʾ����ش��������⣺

��ͼ��X��Һ�� ��
��ʯī�缫�Ϸ����ĵ缫��ӦʽΪ ��
��ԭ��ع���ʱ�������е� (�K������Cl����)���Ͻ���X��Һ�С�
��2����������Ӧ��Ƴɵĵ�����ͼ����ʾ�����ձ��н��������ӵ����ʵ��������ת�Ƶ����ʵ����ı仯��ϵ��ͼ������ش��������⣺
��M�� ���� ��ͼ���еĢ����� �ı仯��
�۵�����ת��Ϊ2 molʱ�������ձ��м��� L 5 mol��L��1 NaOH��Һ����ʹCu2+������ȫ��
��3��������Ҫ�������������(Na2FeO4)��һ����������ˮ�����������кܶ��ŵ㡣
�ٸ���������������֮һ�ǵ�ⷨ����ԭ��ΪFe��2NaOH��2H2O Na2FeO4��3H2��������ʱ�����ĵ缫��Ӧʽ�� ��
Na2FeO4��3H2��������ʱ�����ĵ缫��Ӧʽ�� ��
�ڸ���������������֮������ǿ���Խ�������NaClO����Fe(OH)3���ɸ������ơ��Ȼ��ƺ�ˮ���÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��25 ��ʱ����ʯī�缫���2.0 L 2.5 mol��L��1CuSO4��Һ������0.20 mol���ӷ���ת�ƣ���ش��������⣺
(1)�������� ��Ӧ��
�缫��ӦʽΪ ��
(2)�������� ��Ӧ��
�缫��ӦʽΪ ��
(3)����õ���ͭ�������� ���õ������������ (��״��)����Һ��pH�� ��
(4)����õ�����������ͭƬ����ʯī���缫��������ͭƬ��������� �����Һ��pH ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��ʵ֤��,ԭ����з����ķ�Ӧͨ���Ƿ��ȷ�Ӧ���������л�ѧ��Ӧ������Ƴ�ԭ��ص�������������
A.C(s)+H2O(g) CO(g)+H2(g)����H>0
CO(g)+H2(g)����H>0
B.NaOH(aq)+HCl(aq) NaCl(aq)+H2O(l)����H<0
NaCl(aq)+H2O(l)����H<0
C.2H2(g) +O2(g) 2H2O(l)����H<0
2H2O(l)����H<0
D.CaCO3(s)+2HCl(aq) CaCl2(aq)+H2O(l)+CO2(g)����H<0
CaCl2(aq)+H2O(l)+CO2(g)����H<0
E.CH4(g)+2O2(g) CO2(g)+2H2O(l)����H<0
CO2(g)+2H2O(l)����H<0
(2)��A��B��C��D���ֽ���,��A��B�õ�����������,����������Һ��,B����ʴ����A��D�ֱ�Ͷ������ʵ���Ũ�ȵ�������,D��A��Ӧ���ҡ���ͭ����B������Һ��,�����Ա仯,�����ͭ����C������Һ��,�н���C�������ݴ��ж�A��B��C��D�Ļ����ǿ������˳��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ϻ�ijС���ϣ���ž�սʿΪ��Ѱ�Һ��ʵ�����ˮԴ���Ե���ɽȪˮ���з������飬�����ʾˮ��Ӳ��Ϊ28(����Ӳˮ)����Ҫ�������ӡ�þ���ӡ������Ӻ���������ӡ���˼���������⣺
��1����Ȫˮ����_________Ӳˮ����д����ʱ�������á�����
��2����Ҫ��ȥCa2����Mg2��������ˮ�м���ʯ�Һʹ���Լ�����ʱ�ȼ�________ ���________��ԭ����_____________________________________________________________��
��3��Ŀǰ���������ӽ�����֬��NaR��HR������ˮ����������ʹ��HR��Ϊ�����ӽ�����֬�� ��ˮ�е�Ca2����Mg2���뽻����֬��________�����ӽ������á���ʹ��NaR��Ϊ�����ӽ�����֬��ʧЧ��ɷ���5%��8%_____________��Һ��������
��4�����ϻ������ú�ˮ��������õ�ˮ�������Ǻ�ˮ���õ���������õ�ˮ��ԭ��ͼ����֪��ˮ�к�Na����Cl����Ca2����Mg2����SO42�������ӣ��缫Ϊ���Ե缫��������������⣺
�������ӽ���Ĥ��ָ______________(��A��B)��
��д��ͨ����������ĵ缫��Ӧʽ��_____________________________.
�������������ǣ�_______________________________________________.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com