
���� ��1������m=CVM������Ҫ���ʵ�������
��2����������һ�����ʵ���Ũ����Һ�IJ���ѡ����Ҫ��������
��3����������Ϊ��ʴƷ������ʱӦ����С�ձ����߳���ƿ�У�
��4������ƿ��ʹ�ù�������Ҫ���µߵ���
��5����������һ�����ʵ���Ũ����Һ�IJ�������
��6�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������0.1mol/L��NaOH��Һ500mL����Ҫ���ʵ�����m=0.1mol/L��40g/mol��0.5L=2.0g��
�ʴ�Ϊ��2.0��
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������õ��������У���ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�HIAȱ�ٵ�������ҩ�ס���ͷ�ιܣ�
�ʴ�Ϊ���٢ܢݢޣ�ҩ�ס���ͷ�ιܣ�
��3����������Ϊ��ʴƷ������ʱӦ����С�ձ����߳���ƿ�У�
��ѡ���ۣ�
��4������ƿ��ʹ�ù�������Ҫ���µߵ�������ʹ�ù�����Ӧ�������ƿ�Ƿ�©ˮ��
�ʴ�Ϊ���������ƿ�Ƿ�©ˮ��
��5������һ�����ʵ���Ũ����Һ�IJ��裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ��˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��6����δϴ���ձ������������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��NaOH��Һδ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
�ܳ���NaOH��ʱ��̫�������������������տ�����ˮ��������̼����ȡ���������Ƶ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�ݶ���ʱ���ӿ̶ȣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
��ѡ���ڢݣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ע�������������ͼ��ɣ���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ӵ�ʳ���к��е���أ�KIO3�������Ե�ⷨ�Ʊ�����أ�ʵ��װ����ͼ��ʾ���Ƚ�һ�����ĵ����ڹ�������������Һ��������Ӧ��3I2+6KOH�T5KI+KIO3+3H2O��������Һ��������������������������Һ��������������ʼ��⣮����˵������ȷ���ǣ�������
�ӵ�ʳ���к��е���أ�KIO3�������Ե�ⷨ�Ʊ�����أ�ʵ��װ����ͼ��ʾ���Ƚ�һ�����ĵ����ڹ�������������Һ��������Ӧ��3I2+6KOH�T5KI+KIO3+3H2O��������Һ��������������������������Һ��������������ʼ��⣮����˵������ȷ���ǣ�������| A�� | ��������OH-��a����ͨ�����ӽ���Ĥc����b���� | |
| B�� | ���ŵ����У�KOH��ҺŨ�Ȼ���С | |
| C�� | ��������0.1mol I-�ŵ�ʱ����������6.72LH2 | |
| D�� | a�缫��Ӧʽ��I--6e-+6OH-�TIO3-+3H2O��a������KI����ת��ΪKIO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{64m}{n}$ | B�� | $\frac{32m}{n}$ | C�� | $\frac{n}{32m}$ | D�� | $\frac{n}{64m}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݣ�����Ҫ480mL 1mol•L-1��ϡ���ᣮ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ��
��ͼ����Уʵ���һ�ѧ�Լ�Ũ�����ǩ�ϵIJ������ݣ�����Ҫ480mL 1mol•L-1��ϡ���ᣮ�ø�Ũ���������ˮ���ƣ��ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ��ڲ����������ձ�������Ͳ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
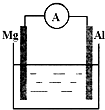 ��ͼ��ʾװ�ã�
��ͼ��ʾװ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʾ������������������Ԫ������Ԫ����ɵ� | |
| B�� | ��ʾ4����ԭ�Ӻ�10����ԭ�� | |
| C�� | ��ʾ2�������������ӣ���4����ԭ�Ӻ�10����ԭ�� | |
| D�� | ��ʾ2�������������ӣ�ÿ���������������к���2����ԭ�Ӻ�5����ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com