
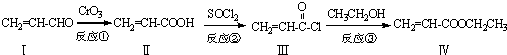
 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ| y |
| 4 |
| z |
| 2 |
 �뻯�����ķ�Ӧ����ṹ��ʽ��
�뻯�����ķ�Ӧ����ṹ��ʽ��| 8 |
| 4 |
| 2 |
| 2 |
| Cu |
| �� |
| Cu |
| �� |
 �뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ��
�뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 3 |
| 2 |
| A��Mg��OH��2��Al��OH��3�����²��ֽ⣬���Կ�����ҵ��ȼ�� |
| B��������Mg��OH��2��Al��OH��3��ȣ�Mg��OH��2��ȼЧ���Ϻ� |
| C��Mg��OH��2��Al��OH��3���ȶ��Ը� |
| D��Mg��OH��2��Al��OH��3��Ϊ��ҵ��ȼ�������Ƿֽ����ȼ�����������������й� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
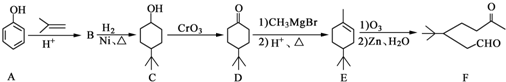
 ����CH2=CH2Ϊԭ���Ʊ��л���
����CH2=CH2Ϊԭ���Ʊ��л���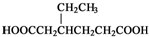 �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���| HBr |
| NaOH��Һ |
| �� |
�鿴�𰸺ͽ���>>
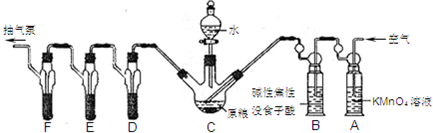
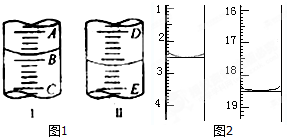
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
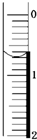 �����Լ������Ƴ������Ƹ�̻�����֯��ȣ���Na2C2O4Ϊ��ɫ���壬����ˮ���������Ҵ����л�ԭ�ԣ�ʵ���ҿ��ñ�KMnO4��Һ�ⶨ���۲�������Na2C2O4�������������������ʲ���KMnO4��Ӧ����
�����Լ������Ƴ������Ƹ�̻�����֯��ȣ���Na2C2O4Ϊ��ɫ���壬����ˮ���������Ҵ����л�ԭ�ԣ�ʵ���ҿ��ñ�KMnO4��Һ�ⶨ���۲�������Na2C2O4�������������������ʲ���KMnO4��Ӧ����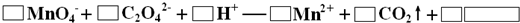
| �ζ��յ� | ��һ���յ� | �ڶ����յ� | �������յ� | ���Ĵ��յ� |
| �ζ���Һ��̶� | 20.72mL | 21.70mL | 20.68mL | 20.70mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
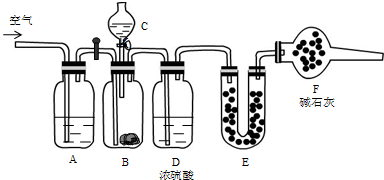
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com