
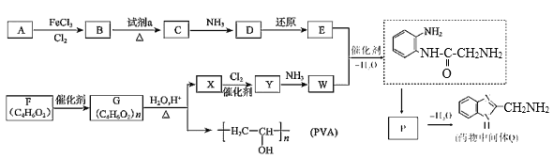
����Ŀ��ҩ���м���Q��ҽ�ò���PVA�ĺϳ�·����ͼ��
��֪����R-Cl+2NH3��R-NH2+NH4Cl
��R-NO2![]() R-NH2
R-NH2
��-NH2+![]()
(1)A�ķ���ʽ��________��
(2)B��C��������Ӧ���Լ�a��________(�����ƣ���
(3)C��Dת���Ļ�ѧ����ʽ��________��
(4)E�Ľṹ��ʽ��________��
(5)F���еĹ�������________(�����ƣ������京����ͬ�����ŵ�ͬ���칹�廹��________�֡�
(6)G��X�Ļ�ѧ����ʽ��________��
(7)W�ܷ������۷�Ӧ���γɵĸ߷��ӽṹ��ʽ��________��
(8)P�Ľṹ��ʽ��________��
���𰸡�C6H6 Ũ���ᡢŨ���� ![]() +2NH3
+2NH3![]()
 +NH4Cl
+NH4Cl 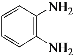 ̼̼˫�������� 4
̼̼˫�������� 4 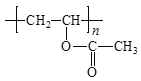 +nH2O
+nH2O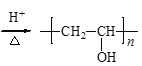 +nCH3COOH
+nCH3COOH 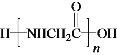
��������
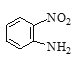
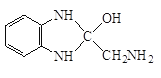
����Q�ṹ��ʽ��A����ʽ֪��AΪ![]() ��A����ȡ����Ӧ����B��BΪ�ȱ���B��C��������Ӧ����C�к�����ԭ�Ӻ�������D������ԭ��Ӧ����E������Q�ṹ��ʽ֪��E�к����������ڵİ�������EΪ
��A����ȡ����Ӧ����B��BΪ�ȱ���B��C��������Ӧ����C�к�����ԭ�Ӻ�������D������ԭ��Ӧ����E������Q�ṹ��ʽ֪��E�к����������ڵİ�������EΪ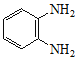 ��DΪ
��DΪ![]() ��CΪ
��CΪ![]() ���Լ�aΪŨ���ᡢŨ���
���Լ�aΪŨ���ᡢŨ���
W�ܷ����ۺϷ�Ӧ�����Q��E�ṹ��ʽ֪��WΪH2NCH2COOH��Y����ȡ����Ӧ����W��X����ȡ����Ӧ����Y��YΪClCH2COOH��XΪCH3COOH��Gˮ������X��PVA����GΪ![]() ��FΪCH3COOCH=CH2��
��FΪCH3COOCH=CH2��
(1)AΪ��������ʽ��C6H6��
(2)B��C��������Ӧ���Լ�a��Ũ���ᡢŨ���
(3) CΪ![]() ��DΪ
��DΪ![]() ��C��DΪȡ����Ӧ���仯ѧ����ʽ��
��C��DΪȡ����Ӧ���仯ѧ����ʽ��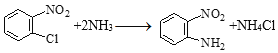 ��
��
(4)E�Ľṹ��ʽ�� ��
��
(5)FΪCH3COOCH=CH2��F���еĹ�������̼̼˫�������������京����ͬ�����ŵ�ͬ���칹�廹��HCOO-C(CH3)=CH2��HCOOCH=CH-CH3��HCOOCH2-CH=CH2��CH2=CH-COOCH3����˹������ֲ�ͬ�ṹ��
(6)GΪ![]() ��FΪCH3COOCH=CH2��G��X�Ļ�ѧ����ʽ�ǣ�
��FΪCH3COOCH=CH2��G��X�Ļ�ѧ����ʽ�ǣ� +nH2O
+nH2O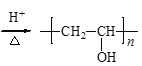 +nCH3COOH��
+nCH3COOH��
(7) WΪH2NCH2COOH��W�����к���-NH2��-COOH������֮���ܷ����ۺϷ�Ӧ���γɵĸ߷��ӽṹ��ʽ��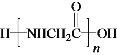 ��
��
(8) 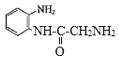 ������-NH2�Ͽ�N-H�����ʻ��Ͽ�C=O˫���������ʻ��ļӳɷ�Ӧ����
������-NH2�Ͽ�N-H�����ʻ��Ͽ�C=O˫���������ʻ��ļӳɷ�Ӧ����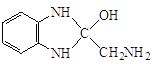 �������ʷ�����ȥ��Ӧ����Q:
�������ʷ�����ȥ��Ӧ����Q: ![]() ������P��
������P�� ��
��


 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ������һ����;�����������������ҽ�Ʊ���Ʒ���Ǻϳ��ڶ�ҩ����м����乤ҵ�ϳɵ�·��֮һ����ͼ��ʾ��

��֪��RCH=CHR1 RCOOH+R1COOH
RCOOH+R1COOH
��ش��������⣺
��1��B�����к��������ŵ�����Ϊ_________��D��ˮ����ķ�Ӧ����Ϊ_________��
��2��Cת��ΪD��ͬʱ����һ���л������ɣ������ʵĽṹ��ʽΪ__________������ת��������B��C��������_________�����ʵ������£�ˮ����ɷ����ۺϷ�Ӧ����һ�ָ߷��ӻ�����û�����Ľṹ��ʽΪ__________��
��3��A��B�Ļ�ѧ����ʽ_________��
��4��D�ж���ͬ���칹�壬��������������ͬ���칹�������_________�֣�д���˴Ź���������4��������ʵĽṹ��ʽ____________��
���DZ��Ķ�Ԫȡ������ܷ���ˮ�ⷴӦ��������Ӧ
��5����1��4-���ȶ���Ϊ����ԭ���Ʊ��Ҷ��ᣬд����Ӧ�ĺϳ�·��ͼ(��ʽΪ��Ӧ��![]() ������) ____________
������) ____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʡ��Ϊ�ҹ���ʳ��ʡ���Թ��ҵ���ʳ��Ӧ������ͻ�����ס�ũ�������кܶ�����ոѣ������������ʣ���ֱ��ȼ�ջ�����������ʽոѵ��ۺ����þ�����Ҫ�����塣�������Խո�Ϊԭ�Ϻϳɾ�����߷��ӻ������·�ߣ�
�ش��������⣺
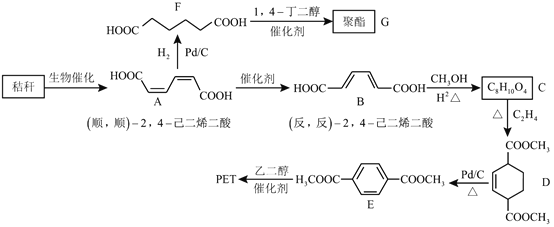
��1�����й��������˵����ȷ����__________��������ĸ��
a���������ζ������![]() ��ͨʽ
��ͨʽ
b����ѿ��ˮ�����ɻ�Ϊͬ���칹��������Ǻ���
c����������Ӧ�����жϵ���ˮ���Ƿ���ȫ
d�����ۺ���ά�ض����ڶ�������Ȼ�߷��ӻ�����
��2��B����C�ķ�Ӧ����Ϊ__________��
��3��D�еĹ���������Ϊ__________��D����E�ķ�Ӧ����Ϊ__________��
��4��F�Ļ�ѧ������__________����F����G�Ļ�ѧ����ʽΪ__________��
��5������һ�ֹ����ŵĶ�ȡ�����㻯����W��E��ͬ���칹�壬![]() W������̼��������Һ��Ӧ����1mol
W������̼��������Һ��Ӧ����1mol![]() ��W����__________�֣����������칹�������к˴Ź�������Ϊ�����Ľṹ��ʽΪ__________��
��W����__________�֣����������칹�������к˴Ź�������Ϊ�����Ľṹ��ʽΪ__________��
��6�����������ϳ�·�ߣ���![]() ,
,![]() ��
��![]() Ϊԭ�ϣ����Լ���ѡ��������Ʊ��Ա�������ĺϳ�·��________________________________________������������ͼ��ʾ��
Ϊԭ�ϣ����Լ���ѡ��������Ʊ��Ա�������ĺϳ�·��________________________________________������������ͼ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ�����������Ӧװ����ͼ�����жԸ�ʵ��������������

A.����������������������
B.ˮԡ�������ŵ������Ⱦ��ȣ����ڿ����¶�
C.�ֲ�Ʒ����������ˮ��5%NaOH��Һϴ�ӣ������������ˮϴ��
D.ϴ�Ӻ�����ˮCaCl2���Ȼ����ˣ��õ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ʵ��С����Ƶ�����ʵ���������


A. ����һ��Ũ�ȵ����� B. �Ʊ���������
C. �Ʊ����ռ�����NO2���� D. �Ʊ������������������ͣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и�������һ�����Դ���������ǣ�������
A.0.1mol/L AgNO3��Һ�У�H+��K+��Cl-��Br-
B.pH=1����Һ�У�Na+��Be2+��Fe2+��NO3-
C.��Al��Ӧ�ų�H2����Һ�У�NH4+��HCO3-��SO42-��NO3-
D.��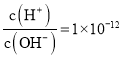 ����Һ�У�Na+��K+��CH3COO-��NO3-
����Һ�У�Na+��K+��CH3COO-��NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������(FeC2O4��2H2O)��һ�ֵ���ɫ��ĩ��ij����С����������װ�ü�����������������ȷֽ�IJ��ֲ��
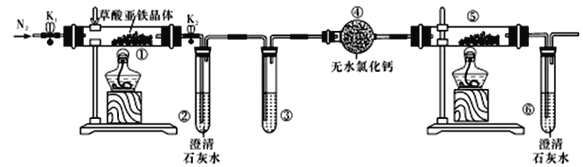
����˵����ȷ����
A. ���ۺ͢��зֱ�ʢ������NaOH��Һ��CuO���ɼ������ɵ�CO
B. ͨ��N2����ҪĿ���Ƿ�ֹ�����е�CO2�Բ���������Ӱ��
C. �������е���ˮCaCl2������ˮ����ͭ�ɼ���ֽ����ɵ�ˮ����
D. ʵ��������е���ɫ��δ��ȫ��ɺ�ɫ�������һ��Ϊ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ͻ�ѧʵ������Ч������Ⱦ����ԼҩƷ����ͼ�У�ijѧ���ڳ���һ�Ű�ֽ�IJ���Ƭ�Ϸ��ñ������ڱ������ϵIJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1mol/L��KBr��KI����������Һ����NaOH(����̪)��FeCl2(��KSCN)��Һ��1�Σ��ڱ��������Ĵ�����2С��KMnO4���壬���μ�һ��Ũ���ᣬ������������Ǻá��ɼ�KMnO4����ܿ��ܽ⣬����������
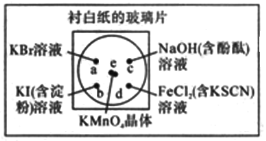
(1)��д����ѧʵ��������MnO2��ȡCl2�Ļ�ѧ����ʽ______________________________��
����ɱ�ʵ������ȡCl2�Ļ�ѧ����ʽ��
___________KMnO4+__________HCl(Ũ)����______KCl+________MnCl2+____Cl2��+______ _______
��÷�Ӧ�����Ļ�ԭ�������ʵ���Ϊ8mol�������ת����ĿΪ_________________��
(2)b����ʵ������Ϊ____________________________________________________��
c����ʵ������Ϊ____________________________________________________��
(3)d����Ӧ�����ӷ���ʽΪ____________________��____________________��
(4)ͨ����ʵ���ܱȽ�Cl2��FeCl3��KMnO4�������������Ե�ǿ��������������ǿ������˳����_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��AgNO3��ˮ��Һ��________________�����������������������������ԣ�����ʱ��pH_____7������>������=������<������ԭ���ǣ������ӷ���ʽ��ʾ����_______________________________________________________________________________________��
ʵ����������AgNO3����Һʱ������AgNO3���������ڽ�Ũ�������У�Ȼ����������ˮϡ�͵������Ũ�ȣ���____________�������ٽ�����������������ˮ�⡣
��2���Ȼ���ˮ��Һ��_______�� ��ԭ���ǣ������ӷ���ʽ��ʾ����_____________________________________________________________ ��
��AlCl3��Һ���ɣ����գ����õ�����Ҫ���������____________________��
��3��������������Һʱ��Ϊ�˷�ֹ����ˮ�⣬���Լ���������______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com