



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ѡ�� | ������ | ������ | �ж� | A | ���ǵؿ��к�����ߵ� ����Ԫ�� |
������������ʹ�õĽ������� | ��ԣ���ԣ��� | B | ����������ϡ���ᷴӦ �������� |
�����������ܻ�ԭ���� ���õ��� |
��ԣ���ԣ��� | C | �����ڹ���Ԫ�� | ��������ijЩ��������������� | �������ԣ��� | D | �ڿ��������ı������� �����ܵ�����Ĥ |
��������������Ӧ | ��ԣ���ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

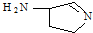 ��������ЦҼ���м�֮��Ϊ
��������ЦҼ���м�֮��Ϊ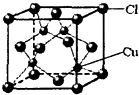 ��ʾ���û�����Ļ�ѧʽΪ
��ʾ���û�����Ļ�ѧʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
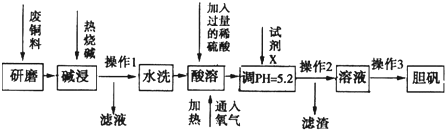
��4�֣��ơ������ֽ������ʼ��仯���������������������Ź㷺��Ӧ�á���ش�
��1�����ֽ����У�����������ˮ��������ǿ�����____���ɴ�˵�����ƵĽ����Ա���____���ǿ������������
��2��ijЩ�������ijɷ����������������ڷ��û������������ʡ��������������Ƿ���ʵ��Լ���____������ţ���
�� ϡ���� �� ʯ����Һ �� KSCN��Һ
��3��������������Һ�е�������������Һ�����ɵĻҰ�ɫ����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ���˹������漰��������ԭ��Ӧ�Ļ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com