
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ����� | |
| ����2��ȡ������Һ���Թ��У��μ�ϡ���ᡣ | |
| ����3��ȡ��������1�еij������Թ��У�________�� | |
| ����4�� | |
 _������Һת��________�У�ϴ�ӣ����ݣ�ҡ�ȡ�
_������Һת��________�У�ϴ�ӣ����ݣ�ҡ�ȡ� ��ܡ����ܡ�������1��0 mol��L��1 Ba��OH��2��Һ��
��ܡ����ܡ�������1��0 mol��L��1 Ba��OH��2��Һ��| ����2�� | ���ְ�ɫ������˵�����Լ�����Ba2������ |
| ����3���μ�ϡ���ᣬ���Ӵ��������ܽ����������嵼�����ʯ��ˮ�� | ����ʯ��ˮ����ǣ�˵�����Լ�����BaCO3[��Դ:] |
| ����4��ȡ����1�е���Һ���ձ��У���pH�Ʋⶨ��pH | pH���Դ���9��6��˵�����Լ�����Ba��OH��2 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
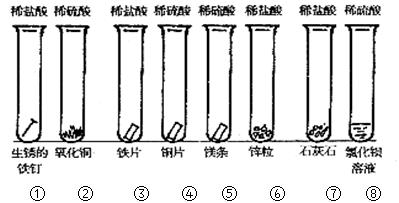
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
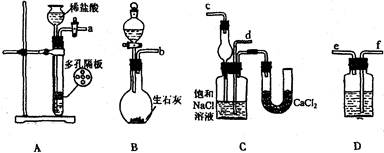
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 250 mL��ƿ�У�����10mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����0.1010 mol��L��1��NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
250 mL��ƿ�У�����10mL 20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min����1~2�η�̪��Һ����0.1010 mol��L��1��NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ� | �ζ����� | ������Һ���/ml | ��NaOH��Һ���������ml�� | |
| �ζ�ǰ/ml | �ζ���/ml | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.30 | 22.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �裬���ų���ȷ�IJ���˳��
�裬���ų���ȷ�IJ���˳��| A��NaOH����ֽ�ϳ������ҳ��ֳ������� |
| B������ǰ����ƿ��������������ˮ |
| C������ʱ���� |
| D��NaOH��Һδ����ȴ��ת�Ƶ�����ƿ��ȥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
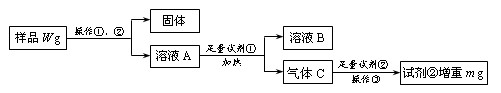
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
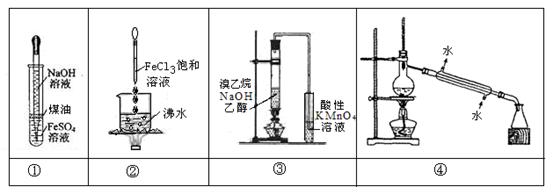
| A��ʵ��٣�������ͷ�ι��е�NaOH��Һ���۲�Fe(OH)2��������ɫ |
| B��ʵ��ڣ����������Һ�����ɫ��ֹͣ���ȣ�������ͨ����ϵʱ���������ЧӦ |
| C��ʵ��ۣ�ͨ���۲�����KMnO4��Һ��ɫ��ȥ��ȷ������ϩ���� |
| D��ʵ��ܣ�����һ���¶ȣ���ʯ�ͷ���Ϊ���͡����͵� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com