
2CuSO4��Na2SO3��2NaCl��Na2CO3===2CuCl����3Na2SO4��CO2��
��CuCl�Ʊ���������Ҫ������������Ϊ20.0%��CuSO4��Һ���Լ������Ƹ���Һ�����CuSO4��5H2O��H2O������֮�ȡ�
��ȷ��ȡ���Ʊ���0.2500 g CuCl��Ʒ����һ������0.5 mol��L��1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20 mL����0.1000 mol��L��1��Ce(SO4)2��Һ�ζ����յ㣬����24.60 mL Ce(SO4)2��Һ���йط���ѧ��ӦΪ
Fe3+��CuCl==Fe2+��Cu2+��Cl- Ce4+��Fe2+==Fe3+��Ce3+
ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���
(1)5��11
(2)��Ʒ��CuCl�������������ϱ�
������������Ҫ�漰�й�һ������������Һ���ƺͼ�ϵʽ�ⷨ�ĵļ���
(1)����ҪCuSO4��5H2O������Ϊx��H2O������Ϊy
CuSO4��5H2O����Է�������Ϊ250��CuSO4����Է�������Ϊ160
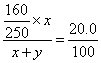
16x=5(x+y)
x��y=5��11
������CuSO4��5H2O��H2O������֮��Ϊ5��11��
(2)����Ʒ��CuCl������Ϊx
�ɻ�ѧ��Ӧ����ʽ��֪��CuCl��Fe2+��Ce4+
![]()
x=0.1000 mol��L-1��24.60��10-3L��99.5 g��mol-1
x=0.2448 g
![]() ��100%=97.92��
��100%=97.92��
97.92%��96.50%
����Ʒ��CuCl�������������ϱ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
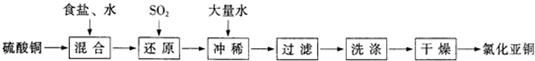
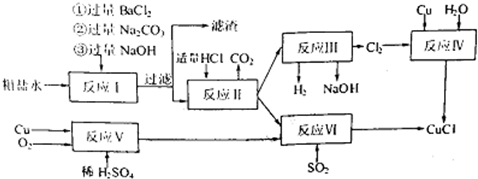
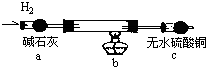
 ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�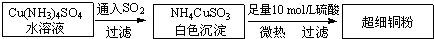
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ��ɫ������ | ���� | ��ɫ������ |
| ������ͭCu��OH��2 | ��ɫ���岻����ˮ | ����ͭ��CuSO4�� | ��Һ����ɫ |
| ������ͭ��CuO�� | ��ɫ���岻����ˮ | �Ȼ�ͭ��CuCl2�� | ��Һ����ɫ��ϡ��Һ����ɫ |
| �Ȼ���ͭ��CuCl�� | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com