

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
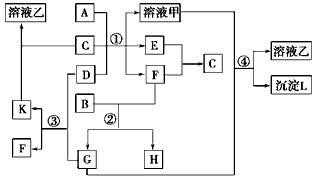
 ��2009?��������ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԣ���
��2009?��������ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

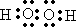
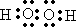
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���
![]()
![]() ��1��A��B��C��D���������ʷֱ�Ϊ���� ������ �������� �������� ���ѧʽ����
��1��A��B��C��D���������ʷֱ�Ϊ���� ������ �������� �������� ���ѧʽ����
![]() ��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�������� ��
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�������� ��
![]() ��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ������� ������ ���ѧʽ����
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ������� ������ ���ѧʽ����
![]() ��4����Ӧ�۲�����K�Ļ�ѧʽΪ���� ����������������������������
��4����Ӧ�۲�����K�Ļ�ѧʽΪ���� ����������������������������
![]() ��5����Ӧ�ܵ����ӷ���ʽΪ���� ��������������������������������������
��5����Ӧ�ܵ����ӷ���ʽΪ���� ��������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���
![]()
![]()
![]()
![]()

��1��A��B��C��D���������ʷֱ�Ϊ �� �� �� ���ѧʽ����
![]() ��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ��� �� ���ѧʽ����
��4����Ӧ�۲�����K�Ļ�ѧʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��09���ľ�����ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���

��1��A��B��C��D���������ʷֱ�Ϊ �� �� �� ���ѧʽ����
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ��� �� ���ѧʽ����
��4����Ӧ�۲�����K�Ļ�ѧʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com