
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ҵ�������Al203ұ��Al |
| B���ý���KMn04��Һ�Ĺ�������ˮ���ͷŵ���ϩ |
| C��ʹ�ô����ı乤ҵ�ϳ�NH3���ղ��� |
| D�����Ǽ���ˮ�������ɷ���������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ��������þ�����Ļ����Ͷ��200mLϡ�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ��������˵������ȷ����
��һ��������þ�����Ļ����Ͷ��200mLϡ�����У�����ȫ���ܽ����������Һ�м���NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ��������˵������ȷ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᣮ
�����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᣮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
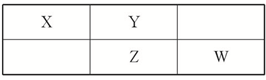 ���ֶ�����Ԫ�������ڱ��е����λ�������ʾ������ZԪ��ԭ�Ӻ��������������������������3����
���ֶ�����Ԫ�������ڱ��е����λ�������ʾ������ZԪ��ԭ�Ӻ��������������������������3�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1LŨ��Ϊ0.1mol/L AlCl3��Һ�к��е���������0.4NA |
| B��3.9g Na2O2����������CO2ʱת�Ƶĵ�������0.05NA |
| C����״���£�3.2gͭ��������Ũ���ᷴӦ���ɵ����庬�еķ�������0.1NA |
| D����״���£�1.12L��SO3������ԭ������0.2NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com