
�ڶ�������֤�����������ɫ�������ܵ�PETG�²��ϣ�PETG�²��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽΪ��
�Իش��������⣺
��1���ϳ�PETG�ĵ�����_________�֣�
��2��ָ����Ӧ���ͣ���_____________��_____________��
��3��д��A��B��I�Ľṹ��ʽ��A________��B____________��I____________��
��4��G���ڷ����ͬ���칹���ж��֣����з��ǻ���Ϊ��λ��ͬ���칹�干��_�֣�
��5����Ӧ���вμӷ�Ӧ��D��E��H�����ʵ���֮��Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�









| NaOH |
| �� |
| NaOH |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

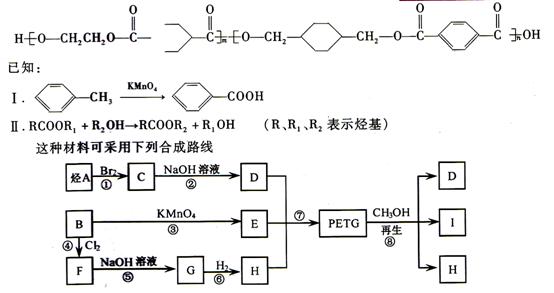
���ֲ��Ͽɲ�������ͼ��ʾ�ĺϳ�·��

![]()
(2)RCOOR1+R2OH![]() RCOOR2+R1OH(R��R1��R2��ʾ����)���÷�ӦΪȡ����Ӧ��������������⣺
RCOOR2+R1OH(R��R1��R2��ʾ����)���÷�ӦΪȡ����Ӧ��������������⣺
(1)�ߵķ�Ӧ������_______________��
(2)д����Ľṹ��ʽ________________________________��
(3)�ϳ�ʱӦ���Ƶĵ�������ʵ�����n(H)��n(E)��n(D)=________��________��________��m��n��ʾ)��
(4)д����Ӧ�ڵĻ�ѧ����ʽ��______________________��
(5)д��ͬʱ������������Ҫ���E������ͬ���칹��Ľṹ��ʽ��
�ٸ�ͬ���칹��ı��������ڵ�����̼ԭ���϶�����ȡ������
�ڸ�ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ��__________��__________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²�
�Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽΪ��
 |
(��)RCOOR�䣫R��OH�D��RCOOR�士R��OH(R��R��R���ʾ����)
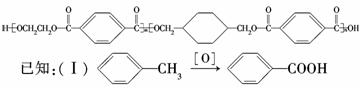
 ���ֲ��Ͽɲ�������·�ߺϳɣ�
���ֲ��Ͽɲ�������·�ߺϳɣ�
�Իش��������⣺
(1)��Ӧ�ڢ�����Լ�X��________����Ӧ�١���������ȡ����Ӧ����________��
(2)д������I�Ľṹ��ʽ��____________________________________________.
(3)д����Ӧ�Ļ�ѧ����ʽ��___________________________________________
______________________________________________________________________.
(4)�ϳ�PETGʱ����������ʵ����ı�����ϵ�ǣ�
n(D)��n(E)��n(H)��________(��m��n��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(18��)�ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ����һ�������ɽ��ջ�����ҵ��չ����˾�����з��ɹ����²��ϣ�����Ϊ������������ء�PETG�Ľṹ��ʽΪ��
��֪��
��1��
��2��RCOOR1 +R2OH RCOOR2+R1OH (R��R1��R2��ʾ����)
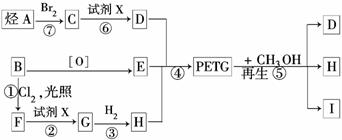
���ֲ��Ͽɲ������ºϳ�·�ߣ�
�Իش��������⣺
��1����Ӧ�ڢ�����Լ�X��______________________
��2���ݵķ�Ӧ������______________________
��3��д���ṹ��ʽ��B��________H��__________I��____________
��4���ϳ�ʱӦ���Ƶĵ�������ʵ���
n(D)��n(E)��n(H)= _____��______��______����m��n��ʾ��
��5��д����ѧ����ʽ��
��Ӧ�ۣ�________________________________________________________
��Ӧ�ޣ�_______________________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com