



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
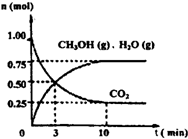 ��2012?������ģ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���
��2012?������ģ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӣ�Ϊ��С������CO2�Ի�����Ӱ�죬һ����������������������ŷ�������һ�����ѧ�Ҽ�ǿ�˶�CO2�������õ��о���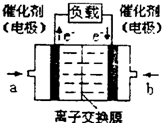
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������KSp(AgCl)=1.8��10-10, KSp(AgI)=1.0��10-16,���������AgCl��AgI�ı�����Һ����Һ��ϣ��������м���һ������AgNO3���壬����˵������ȷ���ǣ� ��
A ����Һ��ϣ�AgCl��AgI������
B ��AgI��Һ����AgNO3��c(Ag+)����KSp(AgI)Ҳ����
C��AgNO3������ AgCl��AgI���ɳ���������AgClΪ��
D��ȡ0.1435��AgCl�������100mLˮ����������仯����c(Cl-)Ϊ0.01mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ��У4�¸��������Ծ������ۣ���ѧ���� ���ͣ�ѡ����
������KSp(AgCl)=1.8��10-10, KSp(AgI)=1.0��10-16�����������AgCl��AgI�ı�����Һ����Һ��ϣ��������м���һ������AgNO3���壬����˵����ȷ����
A������Һ��ϣ�AgCl��AgI������
B����AgI��Һ����AgNO3��c(Ag+)����KSp(AgI)Ҳ����
C����AgNO3������ AgCl��AgI���ɳ���������AgClΪ��
D����ȡ0.1435��AgCl�������100mLˮ����������仯����c(Cl-)Ϊ0.01mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������KSp(AgCl)=1.8��10-10, KSp(AgI)=1.0��10-16�����������AgCl��AgI�ı�����Һ����Һ��ϣ��������м���һ������AgNO3���壬����˵����ȷ����
A������Һ��ϣ�AgCl��AgI������
B����AgI��Һ����AgNO3��c(Ag+)����KSp(AgI)Ҳ����
C����AgNO3������ AgCl��AgI���ɳ���������AgClΪ��
D����ȡ0.1435��AgCl�������100mLˮ����������仯����c(Cl-)Ϊ0.01mol/L
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com