
��ѧ�ϳ���ȼ�շ�ȷ���л�����ɣ����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɡ��л���M(����ʽ��CxHySz)�����ηɻ����������ϵ���Ҫ�ɷ֡�
M���ȼ�յIJ���Ϊ���������д����ѧ����ʽ��_______ _______��
����ʵ�飺
ij��ѧ��ȤС��Ϊ��֤M���Ԫ�ؽ���������ʵ��:��������Ʒ����ȼ�չ�A�У�ͨ��һ����O2���õ�¯����ʹ��ȼ��,����װ����ͼ��ʾ(�г�������װ������ȥ)��
��1����ʵ��װ������˳��Ϊ____________________��������ÿһ������ֻ��ʹ��һ�Σ�
��2��D��ʢ�ŵ��Լ���________
��3����֤���л��ﺬ̼Ԫ�ص�������_________________________________________��
��4��ȼ�չ��з���CuO��������________________________________��
��5��ָ�������д����װ�ã�__________________________________________________��
����ʵ�飺
��1���������CO2���������������ͼ��ʾװ�ã�ʵ�������ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ������ȴ�����£��۶�ȡ��Ͳ��������������������������ȷ˳���ǣ�________������д������ţ���
��2���������CO2�������������ó����������г�������õ���
a��0.1mol/LCaCl2��Һ b��0.1mol/L Ca(OH)2��Һ
c��0.1mol/L Ba(NO3)2��Һ d��0.1mol/L Ba(OH)2��Һ
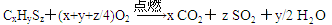
I��1��A��C��B����D����D����B����E
��2�����Ը��������Һ
��3��D����B������Һ����ɫ��E����Һ�����
��4�����л����е�̼Ԫ��ȫ������Ϊ������̼
��5��װ��E���Լ�ƿδ�������ͨ
II��1���ڢ٢ۣ�2��d
��������������������л���M�ķ���ʽΪCxHySz�����ȼ�յIJ���Ϊ���������TCO2��SO2��H2O���ݴ���дȼ�շ���ʽ��
I�����������֪������Ӧ����Ʒ����A��ͨ���������������к���CO2��SO2��H2O�ȣ���Ʒ������Ը��������Һ���鲢����SO2���ó���ʯ��ˮ����CO2������װ������˳��ΪA��C��B��D��E��A��C��D��B��E��D��ʢ�ŵ��Լ������Ը��������Һ��֤���л��ﺬ̼Ԫ�ص�����ΪD����B������Һ����ɫ��E����Һ(����ʯ��ˮ)����ǣ�ȼ�չ��з���CuO�������ǽ��л����е�̼Ԫ��ȫ������Ϊ������̼��װ���еĴ���װ��E���Լ�ƿδ�������ͨ��
II����1���ⶨ����CO2��������������Ӧ��������������ȴ�����£�Ȼ�������Ͳ����Һ��߶�ʹ֮��ͬ�Լ�С������������2�����������Լ��У�bd������CO2���ɳ�����������BaCO3��Ħ������������ⶨ�����Խ�С������ѳ�����Ϊd��
���㣺����ȼ�շ��ⶨ�л������ʽ�����֪ʶ��


 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�㶹����һ����Ȼ���ϣ���ҵ�ϳ���ˮ��ȩ
���������ڴ��������¼��ȷ�Ӧ�Ƶã��������ɼױ�Ϊԭ�������㶹�ص�һ�ֺϳ�·�ߣ����ַ�Ӧ����������������ȥ������֪������Ϣ��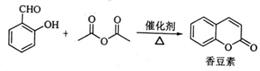
A�������ֲ�ͬ��ѧ�������⣬B����FeCl3��Һ������ɫ��Ӧ��ͬһ��̼ԭ�������������ǻ�ͨ�����ȶ�������ˮ�γɺ�̼��˫���Ľṹ��
��ش��������⣺
��1���㶹�صķ���ʽΪ_______���ɼױ�����A�ķ�Ӧ����Ϊ___________��A�Ľṹ��ʽΪ__________
��2����B����C��Ӧ�Ļ�ѧ����ʽΪ___________ ��
��3��B��ͬ���칹���к��б����Ļ���______�֣� D��ͬ���칹���У����б����Ҽ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ���� ��д�ṹ��ʽ�������б������ܹ��뱥��̼��������Һ��Ӧ�ų�CO2����_________��д�ṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(8��)��0.2molij����һԪ����ȫȼ�պ����ɵ����建��ͨ��ʢ��0.5L 2mol/L������������Һ�У�������������Na2CO3 �� NaHCO3�����ʵ���֮��Ϊ1��3��
��1�����һԪ���ķ���ʽ�� ��2���ô��м��ֽṹ�к��ǻ���ͬ���칹�塣
��3��д����2�����ܱ�����Ϊȩ�Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������һ����;�㷺�ľ�ϸ������Ʒ��ij����С�����ʵ������ȡ���ᴿ���������ķ������£�
��֪�����Ȼ��ƿ����Ҵ��γ�CaCl2��6C2H5OH
���й��л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е�/�� | 34.7 | 78.5 | 118 | 77.1 |
 CH3CH2OCH2CH3��H2O
CH3CH2OCH2CH3��H2O �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNp)�ķ�����ɡ�ȡn g���ְ�������ڴ������г��ȼ�գ�����CO2��H2O��N2���ְ�ͼ��ʾװ�ý���ʵ�飺
��ش������й����⣺
(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������__________________________��
(2)����װ������Ҫ���ȵ�������________(����ĸ���)������ʱӦ�ȵ�ȼ________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��__________________________________________��
(4)װ��D��������___________________________________��
(5)��ȡN2���ʱ��Ӧע�⣺
��________________________________________________��
��________________________________________________��
(6)ʵ���в��N2�����ΪV mL(������Ϊ��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������________(����ĸ���)��
| A������CO2��������� |
| B������H2O������ |
| C��ͨ��O2����� |
| D�����������Է������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�
��֪��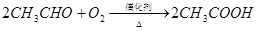
��1����ӦII�Ļ�ѧ����ʽ�� ��
��2��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ�� ��
��3��E������ζ�����ʣ���ʵ��������ͼװ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ�� ���÷�Ӧ����Ϊ ��
�ڸ�װ��ͼ����һ�����ԵĴ����� ��
��4��Ϊ��֤��Ũ�����ڷ�ӦIV�����˴�������ˮ�������ã�ijͬѧ������ͼ�Ľ���װ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ����ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܼ����Լ� | �Թ������Լ� | �л���ĺ��/cm |
| A | 2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1Ũ���� | ����Na2CO3��Һ | 3.0 |
| B | 2 mL�Ҵ���1 mL���� | 0.1 | |
| C | 2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 | 0.6 | |
| D | 2 mL�Ҵ���1 mL���ᡢ���� | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪��CH3CH2OH CH2=CH2��+H20
CH2=CH2��+H20
CH2=CH2+Br2 BrCH2��CH2Br
BrCH2��CH2Br
���Ҵ���1,2-�������顢���ѵ��й������������±���ʾ��
| | �Ҵ� | 1,2-�������� | ���� |
| ͨ��״���µ�״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm-3 | 0.79 | 2.2 | 0.71 |
| �۵�/�� | -130 | 9 | -116 |
| �е�/�� | 78.5 | 132 | 34.6 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����һ��˫����ϩ��������ӳɺ�IJ���ṹ��ʽ��ͼ������������еĽṹ�У� ��
| A��4�� | B��5�� | C��6�� | D��7�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�л���A�ķ���ʽΪC11H16����������Ӧ��ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ�����ⶨ���ݱ����������г��������ⲻ�ٺ���������״�ṹ���ұ�����ֻ��һ�����������ϴ����������Ľṹ��
| A��5�� | B��6�� | C��7�� | D��8�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com