
ŅŃÖŖ£ŗ95”ꏱ£¬KW=1.0”Į10£12.ŌŚøĆĪĀ¶ČĻĀ,²āµĆ0.1mol”¤L£1Na2AČÜŅŗPH=6,ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®H2AŌŚĖ®ČÜŅŗÖŠµÄµēĄė·½³ĢŹ½ĪŖ£ŗH2A 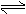 H++HA-£¬HA£
H++HA-£¬HA£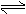 H++A2-
H++A2-
B£®£ØNH4£©2AČÜŅŗÖŠ“ęŌŚĄė×ÓÅØ¶Č¹ŲĻµ£ŗc£ØNH4+£©>c£ØA2-£©>c(H+£©>c£ØOH££©
C£®0.0lmol”¤L-lµÄH2AČÜŅŗpH=2
D£®µČĢå»żµČÅØ¶ČµÄŃĪĖįÓėH2AČÜŅŗ·Ö±šÓė5.6g Zn·“Ó¦£¬H2AČÜŅŗ²śÉśµÄH2¶ą
”¾ÖŖŹ¶µć”æĖ®µÄĄė×Ó»ż³£Źż”¢ČÜŅŗpHÖµ”¢ŃĪČÜŅŗÖŠĄė×ÓÅØ¶Č H1 H2 H3
”¾“š°ø½āĪö”æB½āĪö£ŗøł¾Ż95”ꏱ£¬KW=1.0”Į10£12.ŌŚøĆĪĀ¶ČĻĀ,²āµĆ0.1mol·L£1Na2AČÜŅŗPH=6,æÉÖŖČÜŅŗ³ŹÖŠŠŌ£¬Na2AĪŖĒæĖįĒæ¼īŃĪ”£A”¢H2AĪŖĒæĖįŌŚĖ®ČÜŅŗÖŠĶźČ«µēĄė£¬¹ŹA“ķĪó£»B”¢£ØNH4£©2AĪŖĒæĖįČõ¼īŃĪ£¬ČÜŅŗ³ŹĖįŠŌ£¬ČÜŅŗÖŠ“ęŌŚĄė×ÓÅØ¶Č¹ŲĻµ£ŗc£ØNH4+£©>c£ØA2-£©>c(H+£©>c£ØOH££©£¬¹ŹBÕżČ·£»C”¢0.0lmol·L-lµÄH2AČÜŅŗĒāĄė×ÓÅضČĪŖ0.02mol·L-l£¬pH²»µČÓŚ2£¬¹ŹC“ķĪó£»D”¢µČĢå»żµČÅØ¶ČµÄŃĪĖįÓėH2AČÜŅŗ·Ö±šÓė5.6g Zn·“Ó¦£¬ČōĖį¶¼¹żĮ棬Į½ČÜŅŗ²śÉśµÄH2Ņ»Ńł¶ą£¬¹ŹD“ķĪó”£
¹Ź“š°øŃ”B
”¾Ė¼Ā·µć²¦”æ±¾Ģāæ¼²éĮĖĖ®µÄĄė×Ó»ż³£Źż”¢ČÜŅŗpHÖµ”¢ŃĪČÜŅŗÖŠĄė×ÓÅØ¶ČµČĪŹĢā£¬×ŪŗĻŠŌĒ棬¹Ų¼üŹĒĄūÓĆ95”ꏱ£¬KW=1.0”Į10£12ŗĶŌŚøĆĪĀ¶ČĻĀ0.1mol·L£1Na2AČÜŅŗPH=6,ÅŠ¶ĻH2AŹĒĒæĖį”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķź³ÉĻĀĮŠ¹ŁÄÜĶŵÄŃܱä¹ŲĻµ”£
R—CH2Cl,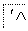
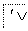
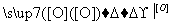
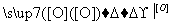
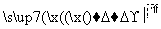 RCOOCH2R
RCOOCH2R
(1)·“Ó¦Ģõ¼ž£ŗ
¢Ł________________________________________________________________________£»
¢Ż________________________________________________________________________”£
(2)½į¹¹¼ņŹ½£ŗ
¢Ś________________________________________________________________________£»
¢Ū________________________________________________________________________£»
¢Ü________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
±ūĻ©ĖįŅŅõ„£Ø»ÆŗĻĪļ¢ō£©ŹĒÖʱøĖÜĮĻ”¢Ź÷Ö¬µČøß¾ŪĪļµÄÖŲŅŖÖŠ¼äĢ壬æÉÓÉĻĀĆęĀ·ĻßŗĻ³É£ŗ
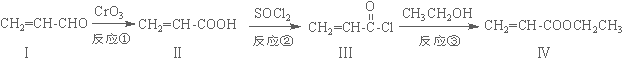 £Ø1£©»ÆŗĻĪļ¢ōµÄ·Ö×ÓŹ½ĪŖ £¬1 mol»ÆŗĻĪļ¢ōĶźČ«Č¼ÉÕĻūŗÄO2ĪŖ mol”£
£Ø1£©»ÆŗĻĪļ¢ōµÄ·Ö×ÓŹ½ĪŖ £¬1 mol»ÆŗĻĪļ¢ōĶźČ«Č¼ÉÕĻūŗÄO2ĪŖ mol”£
£Ø2£©»ÆŗĻĪļ¢ņÄÜŹ¹äåĖ®ĶŹÉ«£¬Ęä·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©·“Ó¦¢ŚŹōÓŚ ·“Ó¦£¬»ÆŗĻĪļ¢ńæÉŅŌÓÉ»ÆŗĻĪļ¢õ£Ø·Ö×ÓŹ½ĪŖC3H6O£©“ß»ÆŃõ»ÆµĆµ½£¬Ōņ»ÆŗĻĪļ¢õ”ś¢ńµÄ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø4£©»ÆŗĻĪļ¢öŹĒ»ÆŗĻĪļ¢ōµÄĶ¬·ÖŅģ¹¹Ģ壬¢öŗ¬ÓŠĢ¼Ģ¼Ė«¼ü²¢ÄÜÓėNaHCO3ČÜŅŗ·“Ó¦·Å³öĘųĢ壬ĘäŗĖ“Ź²ÕńĒāĘ×·åĆ껿֮±ČĪŖ1£ŗ1£ŗ6£¬Ōņ»ÆŗĻĪļ¢öµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø5£©Ņ»¶ØĢõ¼žĻĀ£¬»ÆŗĻĪļ  Ņ²æÉÓė»ÆŗĻĪļ¢ó·¢ÉśĄąĖĘ·“Ó¦¢ŪµÄ·“Ó¦£¬ŌņµĆµ½µÄ²śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
Ņ²æÉÓė»ÆŗĻĪļ¢ó·¢ÉśĄąĖĘ·“Ó¦¢ŪµÄ·“Ó¦£¬ŌņµĆµ½µÄ²śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øÖĢśŗÜČŻŅ×ÉśŠā¶ų±»øÆŹ“£¬ĆæÄźŅņøÆŹ“¶ųĖšŹ§µÄøÖ²ÄÕ¼ŹĄ½ēøÖĢśÄź²śĮæµÄĖÄ·ÖÖ®Ņ»”£
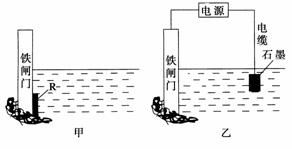
(1)øÖĢśøÆŹ“Ö÷ŅŖŹĒĪüŃõøÆŹ“£¬øĆøÆŹ“¹ż³ĢÖŠµÄµē¼«·“Ó¦Ź½ĪŖ________________________________________________________________________”£
(2)ĪŖĮĖ½µµĶijĖ®æāµÄĢśÕ¢Ćű»øÆŹ“µÄĖŁĀŹ£¬æÉŅŌ²ÉÓĆĶ¼¼×ĖłŹ¾µÄ·½°ø£¬ĘäÖŠŗø½ÓŌŚĢśÕ¢ĆÅÉĻµÄ¹ĢĢå²ÄĮĻRæÉŅŌ²ÉÓĆ________(Ģī×ÖÄø)”£
A£®Ķ B£®ÄĘ C£®Šæ D£®ŹÆÄ«
(3)Ķ¼ŅŅĖłŹ¾µÄ·½°øŅ²æÉŅŌ½µµĶĢśÕ¢ĆŵÄøÆŹ“ĖŁĀŹ£¬ĘäÖŠĢśÕ¢ĆÅÓ¦øĆĮ¬½ÓŌŚÖ±Į÷µēŌ“µÄ________¼«”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĆܱÕČŻĘ÷ÖŠ³äČėµČĪļÖŹµÄĮæµÄĘųĢåAŗĶB£¬Ņ»¶ØĪĀ¶ČĻĀ·¢Éś·“Ó¦:A(g)+x B(g) 2 C(g)“ļµ½Ę½ŗāŗó£¬Ö»øı䷓ӦµÄŅ»øöĢõ¼ž£¬²āµĆČŻĘ÷ÖŠĪļÖŹµÄÅØ¶Č”¢·“Ó¦ĖŁĀŹĖꏱ¼ä±ä»ÆµÄČēĻĀĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÖŠÕżČ·ŹĒ:
2 C(g)“ļµ½Ę½ŗāŗó£¬Ö»øı䷓ӦµÄŅ»øöĢõ¼ž£¬²āµĆČŻĘ÷ÖŠĪļÖŹµÄÅØ¶Č”¢·“Ó¦ĖŁĀŹĖꏱ¼ä±ä»ÆµÄČēĻĀĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÖŠÕżČ·ŹĒ:
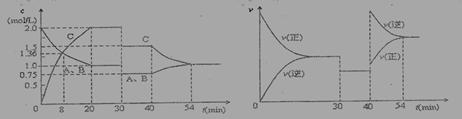
A.30minŹ±½µµĶĪĀ¶Č£¬40minŹ±ÉżøßĪĀ¶Č
B.30minŹ±£¬ĻņČŻĘ÷ÄŚŌŁ³äČėŅ»¶ØĮæµÄC£¬ÖŲŠĀ“ļµ½Ę½ŗāŹ±£¬AµÄĢå»ż·ÖŹż²»±ä
C.·“Ó¦·½³ĢŹ½ÖŠµÄx=1£¬Õż·“Ó¦ĪŖĪüČČ·“Ó¦
D.8minĒ°£¬CµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.08 mol·L-1·min-1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķ¬Ī»ĖŲŹ¾×Ł·ØæÉÓĆÓŚ·“Ó¦»śĄķµÄŃŠ¾æ£¬ĻĀĮŠ·“Ó¦»ņ×Ŗ»ÆÖŠĶ¬Ī»ĖŲŹ¾×Ł±ķŹ¾ÕżČ·µÄŹĒ £Ø £©
A£®2Na218O2 + 2H2O = 4Nal8OH + O2”ü
B£®NH4Cl + 2H2O  NH3·2H2O + HCl
NH3·2H2O + HCl
C£®2KMnO4 + 5H218O2 + 3H2SO4= K2SO4 + 2MnSO4 + 518O2”ü + 8H2O
D£®K37ClO3 + 6HCl = K37Cl + 3Cl2”ü + 3H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀ£¬¶ŌpH=10µÄNaHCO3ČÜŅŗÖŠ£¬ø÷Ī¢Į£µÄĪļÖŹµÄĮæÅØ¶Č·ÖĪöÕżČ·µÄŹĒ£Ø £©
A£®c(CO32—)£¾c(H2CO3)
B£®c(Na£«)£¾c(HCO3—)£¾c(OH”Ŗ)£¾c(H£«)
C£®c(Na£«)£«c(H£«)£½c(HCO3—)£«c(CO32—)£«c(OH”Ŗ)
D£®c(Na£«)£½c(HCO3—)£«c(CO32—)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĘųĢåĪļÖŹµÄÖ÷ŅŖĄ“Ō“¼°¶Ō»·¾³Ó°ĻģĻą¶ŌÓ¦µÄŹĒ
(””””)
| ĘųĢåĪļÖŹ | Ö÷ŅŖĄ“Ō“ | ¶Ō»·¾³µÄÓ°Ļģ | |
| A | ¶žŃõ»ÆĢ¼ | »ÆŹÆČ¼ĮĻµÄČ¼ÉÕ | ĖįÓź |
| B | ¶žŃõ»ÆĮņ | Ęū³µĪ²ĘųµÄÅÅ·Å | ¹ā»ÆѧŃĢĪķ |
| C | ¶žŃõ»ÆµŖ | ¹¤Ņµ·ĻĘųµÄÅÅ·Å | ĪĀŹŅŠ§Ó¦ |
| D | ¼×Č© | ø÷Ąąŗ¬·ÓČ©Ź÷Ö¬½ŗ µÄČĖŌģ°å×°ŹĪ²ÄĮĻ | ŹŅÄŚæÕĘųĪŪČ¾ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓėŹµŃéĻą¹ŲµÄŠšŹöÕżČ·µÄŹĒ_______________”£
A.ÓĆŹÆÄ«µē¼«µē½ā×ćĮæNaClČÜŅŗŅ»¶ĪŹ±¼ä£¬ŅŖ»Öø“µ½ŌČÜŅŗ£¬Ó¦¼ÓČėŅ»¶ØĮæµÄĻ”ŃĪĖį
B. æÉÓĆÖŲ½į¾§·ØĢį“æŗ¬ÉŁĮæNaClµÄKNO3¾§Ģå
C.ÅØĮņĖį²»Š”ŠÄÕ“µ½Ę¤·ōÉĻ£¬Į¢¼“ÓĆĻ”NaOHČÜŅŗĻ“µÓ
D.²ā¶ØijČÜŅŗµÄpHŹ±£¬ŌŚŹŖČóµÄpHŹŌÖ½ÉĻµĪ¼Ó“ż²āČÜŅŗ£¬Ņ»¶ĪŹ±¼äŗó£¬Óė±ź×¼±ČÉ«æضŌ±Č
E.µ°°×ÖŹČÜŅŗÖŠ¼ÓČėĮņĖįĶČÜŅŗ£¬»įÓŠ³ĮµķĪö³ö£¬øĆ³Įµķ²»ÄÜŌŁČÜÓŚĖ®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com