
���п�ͼ�е�A ~ K���ʾ�Ϊ��ѧ�������ʡ���֪B��D��E��L������Ϊ�ܶȱȿ���������壬D��EΪ���ʣ�����Ϊ�����A��I���dz��õ�Ư����F����ɫ��Ӧ�ʻ�ɫ��F��G������L��ˮ��Һ��Ӧ�ų�B�������ͼʾ��ϵ�ش����⣺
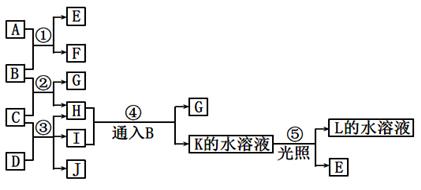
��1��F�������ǣߣߣߣߣߣߣ�K�ĵ���ʽ�ߣߣߣߣߣߡ�
��2����Ӧ��~���У�������������ԭ��Ӧ���ǣߣߣߣߣߣߡ�
��3����Ӧ�ܵ����ӷ���ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ӧ�۵Ļ�ѧ����ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ij��������H��Ӧ����E���÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߡ�
 ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
��






| ��ȼ |
| ��ȼ |
| ��ȼ |
| ��ȼ |
| c |
| 22.4 |
| c |
| 22.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
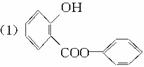
(1)������ͼģ��д�����Ľṹ��ʽ��________________________________________��
(2)����ˮ�⡢���롢�ᴿ�ɵõ������ı��Ӻ�ˮ����(���ǻ�������)�������һ��������˵�����ӡ�̼�ᡢˮ���������������ǿ(�û�ѧ����ʽ��ʾ)��
(3)ͬʱ���������ĸ�Ҫ���ˮ�����ͬ���칹�干��_____________�֡�
�ٺ��б��������ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ������ϡNaOH��Һ�У�1 mol��ͬ���칹������2 mol NaOH������Ӧ����ֻ����������һ�ȴ����
(4)��(3)ȷ����ͬ���칹������ѡһ�֣�ָ��Ϊ���п�ͼ�е�A��

д������������Ӧ�Ļ�ѧ����ʽ(�л����ýṹ��ʽ��ʾ)����ָ����Ӧ�ķ�Ӧ���͡�
��A![]() B______________________________________________________��
B______________________________________________________��
��Ӧ���ͣ�____________________________��
��B+D![]() E__________________________________________________��
E__________________________________________________��
��Ӧ���ͣ�___________________________��
(5)����ˮ����ͱ��ӵĻ������ǵ����ʵ���֮��Ϊn���û������ȫȼ������a L O2��������b g H2O��c L CO2(���������Ϊ��״���µ����)��
�ٷֱ�д��ˮ����ͱ�����ȫȼ�յĻ�ѧ����ʽ(�л�����÷���ʽ��ʾ)��
����������ˮ��������ʵ���Ϊx���г�x�ļ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
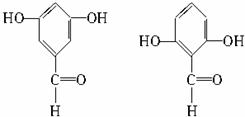
(1)������ͼģ��д�����Ľṹ��ʽ��___________��
(2)����ˮ�⡢���롢�ᴿ�ɵõ������ı��Ӻ�ˮ����(���ǻ�������)�������һ��������˵�����ӡ�̼�ᡢˮ���������������ǿ(�û�ѧ����ʽ��ʾ)
_____________________________________________________________
(3)ͬʱ��������Ҫ���ˮ�����ͬ���칹�干���֡�
�ٺ��б��������ܷ���������Ӧ������ϡNaOH��Һ�У�1mol��ͬ���칹������2molNaOH��Ӧ;��ֻ����������һ�ȴ��
(4)��(3)ȷ����ͬ���칹������ѡһ�֣�ָ��Ϊ���п�ͼ�е�A��
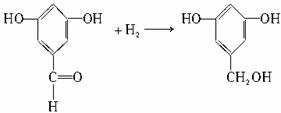
д������������Ӧ�Ļ�ѧ����ʽ(ˮ�����ýṹ��ʽ��ʾ)����ָ����Ӧ�ķ�Ӧ���͡�
��A��B___________________________________________________
��Ӧ����_________��
��B+D��E_________________________________________________
��Ӧ����_________��
(5)����ˮ�����뱽�ӵĻ������ǵ����ʵ���֮��Ϊnmol���û������ȫȼ������O2aL��������bgH2O,cLCO2(���������Ϊ��״���µ����)
�ٷֱ�д��ˮ����ͱ�����ȫȼ�յĻ�ѧ����ʽ(�л�����÷���ʽ��ʾ)
___________________________________________________________
___________________________________________________________
����������ˮ��������ʵ���Ϊxmol���г�x�ļ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)������ͼģ��д�����Ľṹ��ʽ��_________________________________��
(2)����ˮ�⡢���롢�ᴿ�ɵõ������ı��Ӻ�ˮ����(���ǻ�������)�������һ��������˵�����ӡ�̼�ᡢˮ���������������ǿ(�û�ѧ����ʽ��ʾ)��
(3)ͬʱ���������ĸ�Ҫ���ˮ�����ͬ���칹�干��___________�֡��ٺ��б��������ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ������ϡNaOH��Һ�У�1 mol��ͬ���칹������2 mol NaOH������Ӧ����ֻ����������һ�ȴ��
(4)��(3)ȷ����ͬ���칹������ѡһ�֣�ָ��Ϊ���п�ͼ�е�A��

д������������Ӧ�Ļ�ѧ����ʽ(�л����ýṹ��ʽ��ʾ)����ָ����Ӧ�ķ�Ӧ���͡�
��A![]() B__________________����Ӧ���ͣ�_________��
B__________________����Ӧ���ͣ�_________��
��B+D![]() E__________________����Ӧ���ͣ�_________��
E__________________����Ӧ���ͣ�_________��
(5)����ˮ����ͱ��ӵĻ������ǵ����ʵ���֮��Ϊn mol���û������ȫȼ������a L O2��������b g H2O��c L CO2(���������Ϊ��״���µ����)��
�ٷֱ�д��ˮ����ͱ�����ȫȼ�յĻ�ѧ����ʽ(�л�����÷���ʽ��ʾ)��__________________,__________________��
����������ˮ��������ʵ���Ϊx mol���г�x�ļ���ʽ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�人��ʦ��һ���и���������Ӧ�Կ������ۻ�ѧ���� ���ͣ��ۺ���
��15�֣����п�ͼ�е�A ~ K���ʾ�Ϊ��ѧ�������ʡ���֪B��D��E��L������Ϊ�ܶȱȿ���������壬D��EΪ���ʣ�����Ϊ�����A��I���dz��õ�Ư����F����ɫ��Ӧ�ʻ�ɫ��F��G������L��ˮ��Һ��Ӧ�ų�B�������ͼʾ��ϵ�ش����⣺
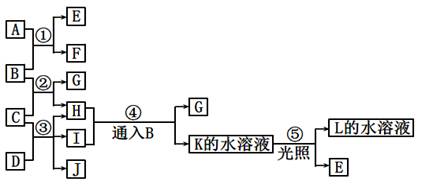
��1��F�������ǣߣߣߣߣߣߣ�K�ĵ���ʽ�ߣߣߣߣߣߡ�
��2����Ӧ��~���У�������������ԭ��Ӧ���ǣߣߣߣߣߣߡ�
��3����Ӧ�ܵ����ӷ���ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ӧ�۵Ļ�ѧ����ʽ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ij��������H��Ӧ����E���÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com