
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
���� ��1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ�������������
��2�����ݼ�����Һʢ���ڼ�ʽ�ζ����У�
��3��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��4���ζ���������Һ�Ӽ�����Һ�����Թ��ɣ���Һ������ʴ�NaOH��NaCl��NaOH���ɣ���������������ΪNaCl������Һʼ�������ԣ����ڵ���غ�ʽ��c��Na+��+c��H+��=c��OH-��+c��Cl-�����ݴ˷����жϣ�
��5���ȷ������������Һ���������Ч�ԣ�Ȼ��������������Һ�����ƽ��ֵ��Ȼ����ݹ�ϵʽHCl��NaOH�����
��6������c�����⣩=$\frac{V��������c������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡ������ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ��������������Ҫ250mL����ƿ����Ͳ���ձ�����ͷ�ι��⣬����һ�ֱ���ʹ�õ������Dz�������
�ʴ�Ϊ����������
��2���ռ��Լ��ԣ�����Ҫ�ü�ʽ�ζ�����ȡ�ռ���Һ���ʴ�Ϊ���
��3���ζ�ǰ��ҺΪ��ɫ���ζ��յ�ʱ��ҺΪ��ɫ�����Եζ��յ�����Ϊ�����һ����Һ�ɺ�ɫ��Ϊ��ɫ������Ӳ���ɫ��
�ʴ�Ϊ�����������һ�����ᣬ��Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��4��A����c��Na+����c��Cl-����c��H+����c��OH-����������c��Na+��+c��H+����c��Cl-��+c��OH-������������Һ����ڵĵ���غ�ʽ���Ǻϣ���A����
B����NaOH��Һֻ�μӼ���ϡ���ᣬ��ʱ��Һֻ��������NaCl��Ӧ�ô��ڣ�c��Na+����c��OH-����c��Cl-����c��H+������B��ȷ��
C����Һʼ�������ԣ����ڵ���غ�ʽ��c��Na+��+c��H+��=c��OH-��+c��Cl-������C��ȷ��
D��c��Na+��+c��H+����c��OH-��+c��Cl-������Һ����ڵĵ��ʽc��Na+��+c��H+��=c��OH-��+c��Cl-����������D����
��ѡBC��
��5�����������Һ������ֱ�Ϊ��19.90mL��20.10mL���������ݾ���Ч�������Һ��ƽ�����Ϊ20.00mL��
HCl��NaOH
1 1
0.2000mol/L��20.00mL C��NaOH����10mL
��ã�C��NaOH��=0.4mol/L����250mL����Һ�к���NaOH������Ϊ��0.4mol/L��0.25L��40g/mol=4.0g���ռ���Ʒ�Ĵ���Ϊ$\frac{4.0g}{4.3g}$��100%=93%��
�ʴ�Ϊ��0.40��93��
��6��A���ζ�ǰƽ�ӣ��ζ����ӣ����V������ƫС������c�����⣩=$\frac{V��������c������}{V�����⣩}$��������֪c�����⣩ƫС���ռ��ƫС����A����
B��δ�ñ�Һ��ϴ�ζ��ܣ���ҺŨ�ȼ�С�����V������ƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$��������֪c�����⣩ƫ���ռ��ƫ��
��B��ȷ��
C���ô���Һ��ϴ��ƿ������Һ�����ʵ���ƫ�����V������ƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$��������֪c�����⣩ƫ���ռ��ƫ��C��ȷ��
D����С�Ľ���Һ������ƿ���棬���V������ƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$��������֪c�����⣩ƫ���ռ��ƫ��D��ȷ��
E���ζ��ӽ��յ�ʱ������������ˮ��ϴ��ƿ�ڱڣ�����Һ�����ʵ������䣬��V��������Ӱ�죬��֪c�����⣩���䣬�ռ�Ȳ��䣬��E����
��ѡBCD��
���� ������Ҫ����������к͵ζ�ʵ��������������������Լ��ζ���������Һ������Ũ�ȴ�С�Ƚϣ��ѶȲ�����ȷд����Һ��ĵ���غ�ʽ�ǽ��ؼ��������ѧ֪ʶ������ɣ�


 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��NO2��������ˮ�У���ɫ��dz��2 NO2��g��?N2O4��g����H��0 | |
| B�� | �ڷ�ӦFeCl3+3KSCN?Fe��SCN��3+3KCl ��ƽ���������KCl���壬ƽ�������ƶ� | |
| C�� | ����֧ʢ��˫��ˮ���Թ��У�һ֧����FeCl3��Һ����Ӧ���Լӿ죬����һ֧�м���CuSO4��Һ����Ӧ�ӿ쵫�����ԣ�˵���������н�ǿѡ���� | |
| D�� | ��MnO2����������˫��ˮ�ķֽ⣬����ΪMnO2�ɽ��÷�Ӧ��ܣ������ӵİٷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ͬ��Ԫ�� | B�� | ����ͬλ�� | C�� | ����ͬ�ֺ��� | D�� | ����ͬ��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
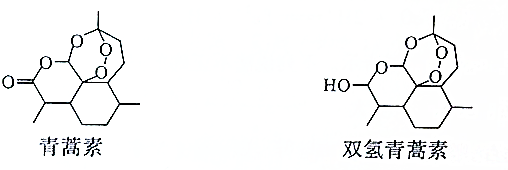
| A�� | ˫�����������֬������ | |
| B�� | �����صķ���ʽΪC15H21O5 | |
| C�� | �����ػ��ϵ�һ��ȡ������11�� | |
| D�� | ������ͨ���ӳɷ�Ӧ����ת��Ϊ˫�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ�����ȩ������������������Ϊȡ����Ӧ | |
| B�� | ��ϩ�ƾ���ϩ�������������������Ӿ۷�Ӧ | |
| C�� | ����������������ˮ���Ϊ��ѧ�仯 | |
| D�� | ʯ�ͷ���ú������ɵõ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
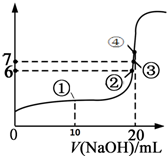 25��ʱ����20mL 0.1mol/L���������Һ����μ���0.1mol/L NaOH��Һ���μӹ����л����Һ��pH�仯������ͼ��ʾ���ش��������⣺
25��ʱ����20mL 0.1mol/L���������Һ����μ���0.1mol/L NaOH��Һ���μӹ����л����Һ��pH�仯������ͼ��ʾ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol��
CH3COO-��aq��+H+��aq����H=+0.3 kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com