

 状元坊全程突破导练测系列答案
状元坊全程突破导练测系列答案 直通贵州名校周测月考直通名校系列答案
直通贵州名校周测月考直通名校系列答案科目:高中化学 来源:不详 题型:实验题
查看答案和解析>>
科目:高中化学 来源:不详 题型:实验题
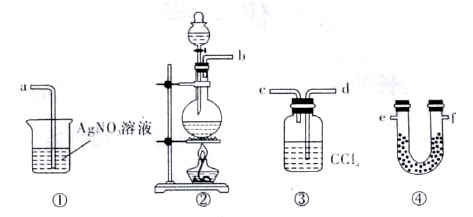
查看答案和解析>>
科目:高中化学 来源:不详 题型:实验题
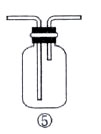
| 物 质 |
| 沸点/℃ | 密度/g·cm-3 | ||
| 乙 醇 | -114 | 78 | 0.789 | ||
| 乙 酸 | 16.6 | 117.9 | 1.05 | ||
| 乙酸乙酯 | -83.6 | 77.5 | 0.900 | ||
| 98%H2SO4 | 10 | 338 | 1.84 |
 从装置Ⅰ中右侧小试管中分离出乙酸乙酯,应进行的操作是:撤出小试管,将混合液倒入 (填仪器名称),用力振
从装置Ⅰ中右侧小试管中分离出乙酸乙酯,应进行的操作是:撤出小试管,将混合液倒入 (填仪器名称),用力振 荡,静置, (填现象),然后将产物从_____口(填“上”或“下”)倒出。
荡,静置, (填现象),然后将产物从_____口(填“上”或“下”)倒出。查看答案和解析>>
科目:高中化学 来源:不详 题型:填空题
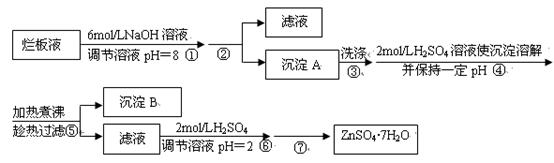
| 离子 | 开始沉淀时的pH | 完全沉淀时的pH |
| Fe3+ | 1.9 | 3.2 |
| Zn2+ | 6.4 | 8.0 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:单选题
| A.用油脂和烧碱制肥皂过程中有水解反应 |
| B.用电石和水反应制乙炔是氧化还原反应 |
| C.用NH4Cl和熟石灰反应制氨气是复分解反应 |
| D.用饱和FeCl3溶液制Fe(OH)3胶体是水解反应 |
查看答案和解析>>
科目:高中化学 来源:不详 题型:填空题

 。
。查看答案和解析>>
科目:高中化学 来源:不详 题型:实验题
 验室准确配制的0.100mol/L的NaOH溶液测定某未知浓度的稀盐酸。其具体实验步骤如下:
验室准确配制的0.100mol/L的NaOH溶液测定某未知浓度的稀盐酸。其具体实验步骤如下:
 形瓶内溶液颜色的变化,当锥形瓶内溶液由
形瓶内溶液颜色的变化,当锥形瓶内溶液由| 实验次数 | 起始滴定管读数 | 终点滴定管读数 |
| 1 | 0.00mL | 24.02mL |
| 2 | 0.50mL | 24.46mL |
| 3 | 1.00mL | 25.02mL |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com