
| ||
| ||
| ||
| ||
| ||
| mg |
| 41n |
| 17m |
| 41n |
| 17m |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� |
| 4100a |
| 22.4w |
| 4100a |
| 22.4w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ�����и���3��������⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
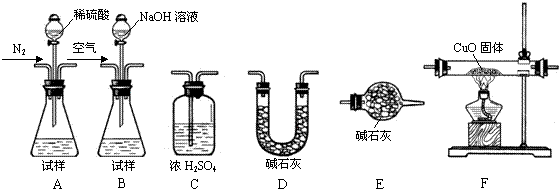
������(AlN)��һ���������ǽ������ϡ�ijAlN��Ʒ������ֺAl2O3���ʣ�Ϊ�ⶨAlN�ĺ����������������ʵ�鷽��������֪��AlN+NaOH+H2O��NaAlO2��NH3����
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��
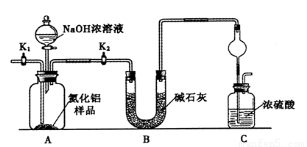
��1����ͼCװ�������θ���ܵ�������______________��
��2���������ʵ�鲽�裺��װ��ʵ��װ�ã�����____________,�ټ���ʵ��ҩƷ����������ʵ�������______________,��Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����______________��
��3������װ�ô���ȱ�ݣ����²ⶨ���ƫ�ߣ�������Ľ����___________��
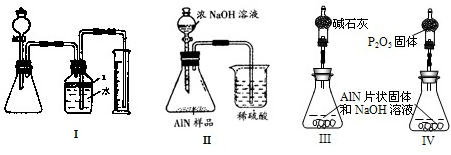
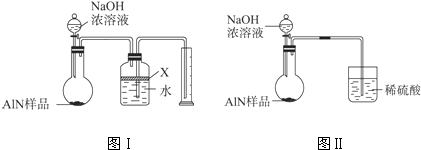
������2������ͼװ�òⶨm g��Ʒ��A1N�Ĵ���(���ּг�װ������ȥ)��
(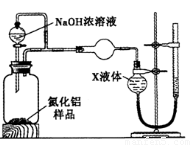
��4��Ϊ�ⶨ������������������װ���е�XҺ�������_________________________��
a��CCl4????????? b��H2O???? c��NH4Cl��Һ??? d��
��5����m g��Ʒ��ȫ��Ӧ�����������������ΪV mL(��ת��Ϊ��״��)����AIN����������__��
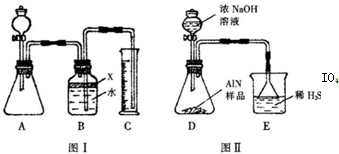
������3�������²���ⶨ��Ʒ��A1N�Ĵ��ȣ�
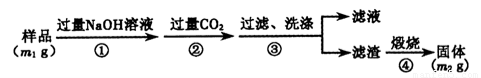
��6����������ɳ��������ӷ���ʽΪ___________________��
��7�����ڲ������δϴ�ӣ��ⶨ�����__________(�ƫ��������ƫ�͡�����Ӱ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com