
“ӻƹ¤³§øõŌüÖŠĢįČ”ĮņĖįÄĘµÄ¹¤ŅÕČēĻĀ£ŗ
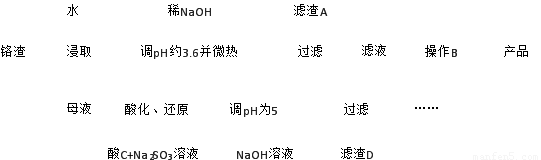
ŅŃÖŖ£ŗ¢ŁøõŌüŗ¬ÓŠNa2SO4¼°ÉŁĮæCr2O72-”¢Fe3+£»¢ŚFe3+”¢Cr3+ĶźČ«³Įµķ£Øc ”Ü1.0”Į10-5 mol”¤L-1£©Ź±pH·Ö±šĪŖ3.6ŗĶ5”£
£Ø1£©”°Ī¢ČČ”±³żÄܼÓæģ·“Ó¦ĖŁĀŹĶā£¬Ķ¬Ź±»¹æÉŅŌ???????? £¬ĀĖŌüAĪŖ ??????? £ØĢī»ÆѧŹ½£©”£
£Ø2£©øł¾ŻČܽā¶Č£ØS£©”×ĪĀ¶Č£ØT£©ĒśĻߣ¬²Ł×÷BµÄ×ī¼Ń·½·ØĪŖ???????? £ØĢī×ÖÄøŠņŗÅ£©
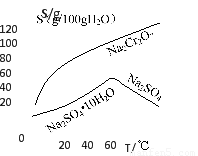
A£®Õō·¢ÅØĖõ£¬³ĆČČ¹żĀĖ
B£®Õō·¢ÅØĖõ£¬½µĪĀ½į¾§£¬¹żĀĖ
£Ø3£©Ėį»ÆŗóCr2O72£æɱ»SO32-»¹Ō³ÉCr3+£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗ????? ????????????????? £»ĖįCĪŖ??????? £¬Cr(OH)3µÄČܶȻż³£ŹżKsp[Cr(OH)3]£½???? ????????? ??? ”£
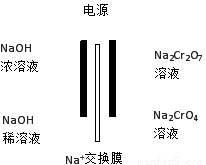
£Ø4£©øł¾Ż2CrO42££«2H+  Cr2O72££«H2OÉč¼ĘĶ¼Ź¾×°ÖĆ£Ø¾łĪŖ¶čŠŌµē¼«£©µē½āNa2CrO4ČÜŅŗÖĘČ”Na2Cr2O7£¬Ķ¼ÖŠÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄ????? ¼«£¬Ęäµē¼«·“Ó¦Ź½ĪŖ?????????? ?????????? ”£
Cr2O72££«H2OÉč¼ĘĶ¼Ź¾×°ÖĆ£Ø¾łĪŖ¶čŠŌµē¼«£©µē½āNa2CrO4ČÜŅŗÖĘČ”Na2Cr2O7£¬Ķ¼ÖŠÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄ????? ¼«£¬Ęäµē¼«·“Ó¦Ź½ĪŖ?????????? ?????????? ”£
£Ø1£©“Ł½ųFe3£«Ė®½āÉś³ÉFe(OH)3¶ų³żČ„£»£Ø2·Ö£©Fe(OH)3£»£Ø2·Ö£©
£Ø2£©A£Ø2·Ö£©
£Ø3£©3SO32-£«Cr2O72-£«8H£«£½2Cr3£«£«3SO42-£«4H2O£»£Ø3·Ö£¬»ÆѧŹ½ŗĶÅäĘ½“ķĪó0·Ö£©
H2SO4£»£Ø2·Ö£©1.0”Į10£32 mol4”¤L-4£Ø2·Ö£¬²»“ųµ„Ī»æŪ1·Ö£©
£Ø4£©Õż¼«£¬£Ø2·Ö£©4OH£-4e££½O2”ü£«2H2O£Ø2·Ö£©£ØĪŽ”°”ü”±æŪ·Ö1·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©ŃĪĄąµÄĖ®½ā·“Ó¦ĪŖĪüČČ·“Ó¦£¬ĖłŅŌĪ¢ČČÄÜ“Ł½ųFe3£«Ė®½āÉś³ÉFe(OH)3¶ų³żČ„£»Fe3+Ė®½āÉś³ÉFe(OH)3³Įµķ£¬ĖłŅŌĀĖŌüAĪŖFe(OH)3”£
£Ø2£©øł¾ŻČܽā¶Č£ØS£©”×ĪĀ¶Č£ØT£©ĒśĻߣ¬æÉŅŌ擳öĪĀ¶Č½Ļøߏ±£¬Ėę×ÅĪĀ¶ČµÄÉżøߣ¬Na2SO4µÄČܽā¶ČÖš½„¼õŠ”£¬ĖłŅŌ²ÉÓĆÕō·¢ÅØĖõ£¬³ĆČČ¹żĀĖµÄ·½·Ø£¬¹ŹAĻīÕżČ·”£
£Ø3£©Ėį»ÆŗóCr2O72£°ŃSO32-Ńõ»ÆĪŖSO42?£¬ĖłŅŌĄė×Ó·½³ĢŹ½ĪŖ£ŗ3SO32-£«Cr2O72-£«8H£«£½2Cr3£«£«3SO42-£«4H2O£»ŅņĪŖ×īÖÕ²śĘ·ĪŖNa2SO4£¬ĪŖ±ÜĆā²śÉśŌÓÖŹ£¬ĖįCĪŖH2SO4£»Ē”ŗĆĶźČ«³ĮµķŹ±Ąė×ÓÅضČĪŖ1.0”Į10-5 mol”¤L-1£¬Cr3+ĶźČ«³ĮµķŹ±pHĪŖ5£¬c(OH?)= 1.0”Į10-9 mol”¤L-1£¬ĖłŅŌCr(OH)3µÄČܶȻż³£ŹżKsp[Cr(OH)3]£½c(Cr3+)?c3(OH?)= 1.0”Į10-5 mol”¤L-1”Į(1.0”Į10-9 mol”¤L-1)3=1.0”Į10£32 mol4”¤L-4”£
£Ø4£©øł¾ŻŹ¾ŅāĶ¼£¬Ķ¼ÖŠÓŅ²ąNa2CrO4×Ŗ»ÆĪŖNa2Cr2O7£¬ŠčŅŖH+£¬ĖµĆ÷ÓŅ²ąµē¼«·¢ÉśH2OµēĄė²śÉśµÄOH?·Åµē£¬Ź¹H2OµÄµēĄėĘ½ŗāĻņÓŅŅĘ¶Æ£¬H+Ōö¶ą£¬ĖłŅŌÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄÕż¼«£¬µē¼«·½³ĢŹ½ĪŖ£ŗ4OH£-4e££½O2”ü£«2H2O
æ¼µć£ŗ±¾Ģāæ¼²é»Æѧ¹¤ŅÕĮ÷³Ģ·ÖĪö”¢¾§Ģå½į¾§”¢Ąė×Ó·½³ĢŹ½µÄŹéŠ“”¢ČܶȻż³£ŹżµÄ¼ĘĖć”¢µē½āŌĄķ¼°Ó¦ÓĆ”£


 ³å“Ģ100·Öµ„ŌŖÓÅ»ÆĮ·æ¼¾ķĻµĮŠ“š°ø
³å“Ģ100·Öµ„ŌŖÓÅ»ÆĮ·æ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø10·Ö£©ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾ŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬ĻĀĶ¼ŹĒij¹¤³§“Óŗ£Ė®ÖŠĢįČ”Ć¾µÄÖ÷ŅŖ²½Öč”£Ń§Éś¾ĶÕāøöæĪĢāÕ¹æŖĮĖĢÖĀŪ”£Ń§Éś¾ĶÕāøöæĪĢāĢį³öĮĖŅŌĻĀĪŹĢā£ŗ
£ØŅ»£©ŌŚŗ£Ė®ĢįĆ¾µÄ¹ż³ĢÖŠČēŗĪŹµĻÖ¶ŌĆ¾Ąė×ÓµÄø»¼Æ
ÓŠČżøöѧɜĢį³ö×Ō¼ŗµÄ¹Ūµć”£
ѧɜ¼×µÄ¹Ūµć£ŗÖ±½ÓĶłŗ£Ė®ÖŠ¼ÓČė³Įµķ¼Į”£
ѧɜŅŅµÄ¹Ūµć£ŗøßĪĀ¼ÓČČÕō·¢ŗ£Ė®ŗó£¬ŌŁ¼ÓČė³Įµķ¼Į”£
ѧɜ±ūµÄ¹Ūµć£ŗĄūÓĆɹŃĪŗóµÄæąĀ±Ė®£¬ŌŁ¼ÓČė³Įµķ¼Į”£
Ķعż·ÖĪö±Č½ĻÄćČĻĪŖѧɜ µÄ¹ŪµćÕżČ·£ØĢīѧɜŠņŗÅ£©£¬¼ņŹöĄķÓÉ£ŗ ”£
£Ø¶ž£©ŌŚŗ£Ė®ĢįĆ¾µÄ¹ż³ĢÖŠČēŗĪŹµĻÖ¶ŌĆ¾Ąė×ӵķ֥ė£æ
£Ø1£©ĪŖĮĖŹ¹Ć¾Ąė×Ó³ĮµķĻĀĄ“£¬²¢³ä·ÖĄūÓƵ±µŲµÄ±“æĒ£ØÖ÷ŅŖ³É·ÖĪŖĢ¼ĖįøĘ£©×ŹŌ“£¬¼ÓČėµÄ×ćĮæŹŌ¼Į¢ŁŹĒ £ØĢī»ÆѧŹ½£©”£
£Ø2£©¼ÓČėŹŌ¼Į¢Łŗó£¬Äܹ»·ÖĄėµĆµ½Mg£ØOH£©2³ĮµķµÄ·½·ØŹĒ £ØĢī±źŗÅ×ÖÄø£©
A£®ÕōĮó B£®¹żĀĖ C£®ŻĶČ” D£®·ÖŅŗ
£Ø3£©¼ÓČėµÄ×ćĮæŹŌ¼Į¢ŚŹĒ £ØĢī»ÆѧŹ½£©”£
£Ø4£©Š“³öÓÉĪŽĖ®MgCl2ÖĘČ”½šŹōĆ¾µÄ»Æѧ·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗŚĮś½Ź”Ėē±õŅ»ÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ÄæĒ°ŹĄ½ēÉĻ60%µÄĆ¾ŹĒ“Óŗ£Ė®ÖŠĢįČ”µÄ£¬ĻĀĶ¼ŹĒij¹¤³§“Óŗ£Ė®ÖŠĢįČ”Ć¾µÄÖ÷ŅŖ²½Öč”£Ń§Éś¾ĶÕāøöæĪĢāÕ¹æŖĮĖĢÖĀŪ”£Ń§Éś¾ĶÕāøöæĪĢāĢį³öĮĖŅŌĻĀĪŹĢā£ŗ £ØŅ»£©ŌŚŗ£Ė®ĢįĆ¾µÄ¹ż³ĢÖŠČēŗĪŹµĻÖ¶ŌĆ¾Ąė×ÓµÄø»¼Æ
£ØŅ»£©ŌŚŗ£Ė®ĢįĆ¾µÄ¹ż³ĢÖŠČēŗĪŹµĻÖ¶ŌĆ¾Ąė×ÓµÄø»¼Æ
ÓŠČżøöѧɜĢį³ö×Ō¼ŗµÄ¹Ūµć”£ ѧɜ¼×µÄ¹Ūµć£ŗÖ±½ÓĶłŗ£Ė®ÖŠ¼ÓČė³Įµķ¼Į”£
ѧɜ¼×µÄ¹Ūµć£ŗÖ±½ÓĶłŗ£Ė®ÖŠ¼ÓČė³Įµķ¼Į”£ ѧɜŅŅµÄ¹Ūµć£ŗøßĪĀ¼ÓČČÕō·¢ŗ£Ė®ŗó£¬ŌŁ¼ÓČė³Įµķ¼Į”£
ѧɜŅŅµÄ¹Ūµć£ŗøßĪĀ¼ÓČČÕō·¢ŗ£Ė®ŗó£¬ŌŁ¼ÓČė³Įµķ¼Į”£ ѧɜ±ūµÄ¹Ūµć£ŗĄūÓĆɹŃĪŗóµÄæąĀ±Ė®£¬ŌŁ¼ÓČė³Įµķ¼Į”£
ѧɜ±ūµÄ¹Ūµć£ŗĄūÓĆɹŃĪŗóµÄæąĀ±Ė®£¬ŌŁ¼ÓČė³Įµķ¼Į”£ Ķعż·ÖĪö±Č½ĻÄćČĻĪŖѧɜ µÄ¹ŪµćÕżČ·£ØĢīѧɜŠņŗÅ£©£¬¼ņŹöĄķÓÉ£ŗ
Ķعż·ÖĪö±Č½ĻÄćČĻĪŖѧɜ µÄ¹ŪµćÕżČ·£ØĢīѧɜŠņŗÅ£©£¬¼ņŹöĄķÓÉ£ŗ  ”£
ӣ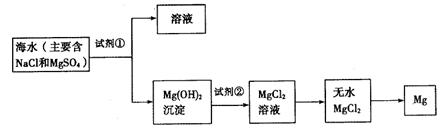


 £Ø¶ž£©ŌŚŗ£Ė®ĢįĆ¾µÄ¹ż³ĢÖŠČēŗĪŹµĻÖ¶ŌĆ¾Ąė×ӵķ֥ė£æ
£Ø¶ž£©ŌŚŗ£Ė®ĢįĆ¾µÄ¹ż³ĢÖŠČēŗĪŹµĻÖ¶ŌĆ¾Ąė×ӵķ֥ė£æ £Ø1£©ĪŖĮĖŹ¹Ć¾Ąė×Ó³ĮµķĻĀĄ“£¬²¢³ä·ÖĄūÓƵ±µŲµÄ±“æĒ£ØÖ÷ŅŖ³É·ÖĪŖĢ¼ĖįøĘ£©×ŹŌ“£¬¼ÓČėµÄ×ćĮæŹŌ¼Į¢ŁŹĒ £ØĢī»ÆѧŹ½£©”£
£Ø1£©ĪŖĮĖŹ¹Ć¾Ąė×Ó³ĮµķĻĀĄ“£¬²¢³ä·ÖĄūÓƵ±µŲµÄ±“æĒ£ØÖ÷ŅŖ³É·ÖĪŖĢ¼ĖįøĘ£©×ŹŌ“£¬¼ÓČėµÄ×ćĮæŹŌ¼Į¢ŁŹĒ £ØĢī»ÆѧŹ½£©”£ £Ø2£©¼ÓČėŹŌ¼Į¢Łŗó£¬Äܹ»·ÖĄėµĆµ½Mg£ØOH£©2³ĮµķµÄ·½·ØŹĒ £ØĢī±źŗÅ×ÖÄø£©
£Ø2£©¼ÓČėŹŌ¼Į¢Łŗó£¬Äܹ»·ÖĄėµĆµ½Mg£ØOH£©2³ĮµķµÄ·½·ØŹĒ £ØĢī±źŗÅ×ÖÄø£©
| A£®ÕōĮó | B£®¹żĀĖ | C£®ŻĶČ” | D£®·ÖŅŗ |
 £Ø3£©¼ÓČėµÄ×ćĮæŹŌ¼Į¢ŚŹĒ £ØĢī»ÆѧŹ½£©”£
£Ø3£©¼ÓČėµÄ×ćĮæŹŌ¼Į¢ŚŹĒ £ØĢī»ÆѧŹ½£©”£ £Ø4£©Š“³öÓÉĪŽĖ®MgCl2ÖĘČ”½šŹōĆ¾µÄ»Æѧ·½³ĢŹ½
£Ø4£©Š“³öÓÉĪŽĖ®MgCl2ÖĘČ”½šŹōĆ¾µÄ»Æѧ·½³ĢŹ½ ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ°²»ÕŹ”ŗĻ·ŹŹŠøßČżÉĻѧʌµŚŅ»“Ī½Ģѧ֏Įæ¼ģ²ā»ÆѧŹŌĢā£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©2011Äź8ŌĀŌĘÄĻ±ØµĄøõŌüĪŪČ¾ŹĀ¼ž£¬øõŌüÖŠµ½ŗ¬Į§+6¼ŪµÄøõ¶ųŹ¹Ę䶾ŠŌĒæĄūÓĆīŃĢśæó£ØTiO2”¢FeO”¢Fe2O3£©Éś²śīŃ°×·ŪµÄø±²śĘ·£ØĮņĖįŃĒĢś£©£¬æÉŅŌ°ŃĘ仹ŌĪŖ+3¼ŪµÄøõ£¬ŌŚ¼īŠŌĢõŹ²Éś³ÉĒāŃõ»Æøõ³Įµķ”£
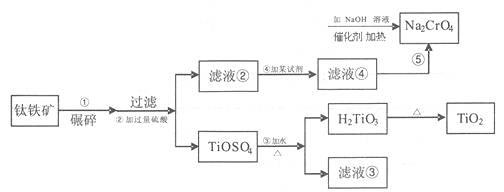
£Ø1£©ÄėĖéµÄÄæµÄŹĒ
£Ø2£©¼ÓČė¹żĮæµÄĮņĖį³żČĆĢśæó³ä·Ö·“Ó¦Ķā£¬»¹Ęšµ½µÄ×÷ÓĆŹĒ [Ą“
£Ø3£©µŚ¢Ü²½Ėł¼ÓŹŌ¼ĮŹĒ
£Ø4£©ÉĻŹöĪļÖŹÖŠæÉŃ»·Ź¹ÓƵďŌ¼ĮŹĒ£ØŠ“»ÆѧŹ½£©
£Ø5£©Š“³öĀĖŅŗ¢ÜÓėNa2CrO4ŌŚNaOHČÜŅŗÖŠ·“Ó¦µÄĄė×Ó·“Ó¦·½³ĢŹ½
£Ø6£©µŚ¢Ū²½ÖŠ¼ÓČȵÄÄæµÄŹĒ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com