������A��ϵͳ����Ϊ1��4-����-2-��ϩ����AD�ṹ��ʽΪ��BrCH
2CH=CHCH
2Br����C
4H
6ΪCH
2=CH-CH
2=CH
2��A���������Ƶ�ˮ��Һ��������B����BΪHOCH
2CH=CHCH
2OH��B��HBr����C����CΪHOCH
2CH
2CHBrCH
2OH��C�����Ը��������Һ������D��HOOCCH
2CHBrCOOH��D���������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ����E��HOOCCH=CHCOOH����Ӧ��Ϊȡ����Ӧ�����Ϸ�Ӧ�ڵIJ����֪����ʽΪC
13H
9NO
4S�����ʵĽṹ��ʽΪ��

���ݴ˽��н��
���
�⣺A��ϵͳ����Ϊ1��4-����-2-��ϩ����A�ṹ��ʽΪ��BrCH
2CH=CHCH
2Br����C
4H
6ΪCH
2=CH-CH
2=CH
2��A���������Ƶ�ˮ��Һ��������B����BΪHOCH
2CH=CHCH
2OH��B��HBr����C����CΪHOCH
2CH
2CHBrCH
2OH��C�����Ը��������Һ������D��HOOCCH
2CHBrCOOH��D���������ƵĴ���Һ�м��ȷ�����ȥ��Ӧ����E��HOOCCH=CHCOOH����Ӧ��Ϊȡ����Ӧ�����Ϸ�Ӧ�ڵIJ����֪��C
13H
9NO
4S�ṹ��ʽΪ��

��
��1�����ݷ�����֪������ʽΪC
13H
9NO
4S�Ľṹ��ʽΪ��

���ʴ�Ϊ��

��
��2��
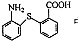
�����к��й�����Ϊ��-S-���������Ȼ����ʴ�Ϊ���������Ȼ���
��3����Ӧ�ܵĻ�ѧ����ʽΪ��BrCH
2CH=CHCH
2Br+2NaOH
2NaBr+HOCH
2CH=CHCH
2OH������̼̼˫�����ױ����Ը��������Һ���������Է�Ӧ�ݵ�Ŀ���DZ���̼̼˫������ֹ�����Ը��������Һ������
�ʴ�Ϊ��BrCH
2CH=CHCH
2Br+2NaOH
2NaBr+HOCH
2CH=CHCH
2OH������̼̼˫������ֹ�����Ը��������Һ������
��4��B�Ľṹ��ʽΪ��HOCH
2CH=CHCH
2OH�����ܷ���������Ӧ�������к���ȩ��������NaOH��Һ�ܷ�Ӧ����NaHCO
3��Һ����Ӧ��������к��������������Ȼ����ۺ˴Ź�������ֻ������壬�������ֻ����3�ֵ�ЧHԭ�ӣ������������л���Ľṹ��ʽΪ��
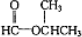
��
�ʴ�Ϊ��
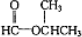
��
��5����֪��һSH���ϻ�����������-OH���ƣ���
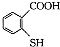
��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ��

��
�ʴ�Ϊ��

��
��6����֪��Ӧԭ��
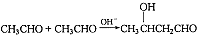
�����Ҵ�Ϊԭ����ȡCH
2=CH-CH=CH
2�������Ƚ��Ҵ�����������CH
3CHO��Ȼ������Ӧ
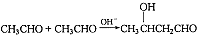
�����ɵ�

�����������ӳɷ�Ӧ����

��

��Ũ���������¼��ȷ�����ȥ��Ӧ����CH
2=CH-CH=CH
2�����Է�Ӧ����Ϊ��CH
3CH
2OH
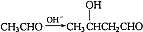

CH
2=CH-CH=CH
2��
�ʴ�Ϊ��CH
3CH
2OH
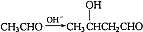

CH
2=CH-CH=CH
2��

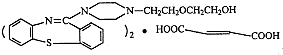 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�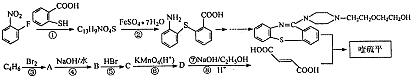
 �г����Ѽ���һSһ���⣬�����еĹ���������Ϊ
�г����Ѽ���һSһ���⣬�����еĹ���������Ϊ ��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ
��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ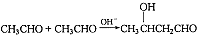 ����ƺ������������Ҵ���ȡ���������е�C4H6��������ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ��������
����ƺ������������Ҵ���ȡ���������е�C4H6��������ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ��������
 ���ݴ˽��н��
���ݴ˽��н�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� �����к��й�����Ϊ��-S-���������Ȼ����ʴ�Ϊ���������Ȼ���
�����к��й�����Ϊ��-S-���������Ȼ����ʴ�Ϊ���������Ȼ���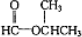 ��
��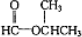 ��
�� ��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ��
��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�� ��
��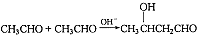 �����Ҵ�Ϊԭ����ȡCH2=CH-CH=CH2�������Ƚ��Ҵ�����������CH3CHO��Ȼ������Ӧ
�����Ҵ�Ϊԭ����ȡCH2=CH-CH=CH2�������Ƚ��Ҵ�����������CH3CHO��Ȼ������Ӧ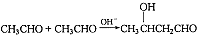 �����ɵ�
�����ɵ� �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ���� ��
�� ��Ũ���������¼��ȷ�����ȥ��Ӧ����CH2=CH-CH=CH2�����Է�Ӧ����Ϊ��CH3CH2OH
��Ũ���������¼��ȷ�����ȥ��Ӧ����CH2=CH-CH=CH2�����Է�Ӧ����Ϊ��CH3CH2OH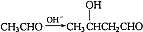

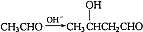




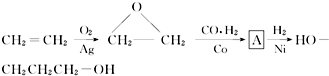
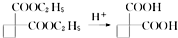 ��������Ƴ������ķ�Ӧ����ͼ��
��������Ƴ������ķ�Ӧ����ͼ��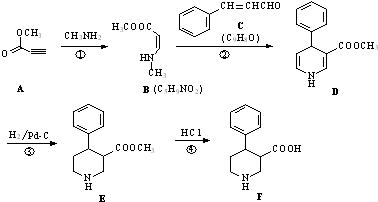
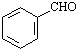 ��CH3CH2OH��
��CH3CH2OH��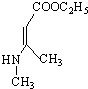 Ϊԭ�ϣ��ϳ�
Ϊԭ�ϣ��ϳ�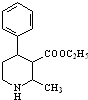 ��д���ϳ�����ͼ�����Լ����ã����ϳ�����ͼʾ�����£�CH2�TCH2
��д���ϳ�����ͼ�����Լ����ã����ϳ�����ͼʾ�����£�CH2�TCH2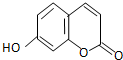 �������㶹���������п����������ã��û��������㶹�أ�
�������㶹���������п����������ã��û��������㶹�أ�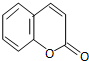 �������������4-�ǻ�ˮ��ȩ���������Ƶã�
�������������4-�ǻ�ˮ��ȩ���������Ƶã�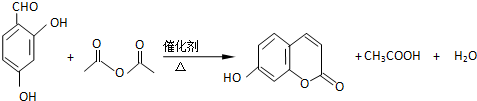
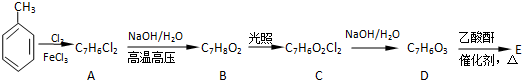
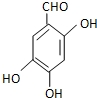 ����������Ӧ�Ƶã���д���÷�Ӧ�Ļ�ѧ����ʽ
����������Ӧ�Ƶã���д���÷�Ӧ�Ļ�ѧ����ʽ ����Ϊ��������-����������Ӧ�����ⷴӦ�ںϳɳ�������ʱ���źܴ�����ã������졢�Ƶȳ��������ĺϳɾ���˵����Щ��Ӧ���ձ��ԣ�
����Ϊ��������-����������Ӧ�����ⷴӦ�ںϳɳ�������ʱ���źܴ�����ã������졢�Ƶȳ��������ĺϳɾ���˵����Щ��Ӧ���ձ��ԣ�
 ��
�� ����������I�У��л���W������Ϊ
����������I�У��л���W������Ϊ
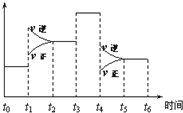 һ�ܱ���ϵ�з�����Ӧ��2SO2��g��+O2��g��?2SO3��g������ͼ��ʾ�÷�Ӧ�����ʣ�v����ijһʱ�䣨t�����ڵı仯��������ʱ����У�SO3�İٷֺ�����ߵ��ǣ�������
һ�ܱ���ϵ�з�����Ӧ��2SO2��g��+O2��g��?2SO3��g������ͼ��ʾ�÷�Ӧ�����ʣ�v����ijһʱ�䣨t�����ڵı仯��������ʱ����У�SO3�İٷֺ�����ߵ��ǣ�������