
��18�֣��Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)�����������������õ�ⷨ����WC���Ʊ�CoxOy���Ĺ������̼�ͼ���£�
��1�����ʱ�Ͼɵ�����������������������������������______��
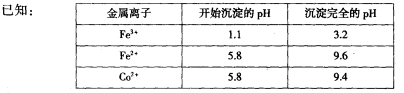
��2��ͨ�˰�����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ��pH���Χ��____________��
��3��ʵ����NH4HCO3��Һ�Լ��ԣ��Ʊ�CoCO3ʱ��ѡ�õļ��Ϸ�ʽ��_______������ţ���ԭ����_______��
a������Һ��NH4HCO3��Һͬʱ���뵽��Ӧ������
b������Һ�������뵽ʢ��NH4HCO3��Һ�ķ�Ӧ������
c����NH4HCO3��Һ�������뵽ʢ����Һ�ķ�Ӧ������
д������CoCO3�����ӷ���ʽ______________________________________��
��4��ʵ���л�õ���ϴ�Ӳ���֣��ڱ���ʱ�������Ⱦ�����壬����Ⱦ������ijɷ�Ϊ_______________���ѧʽ����
��5��ʵ����������װ����ȡ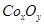 �����ⶨ�仯ѧʽ��
�����ⶨ�仯ѧʽ��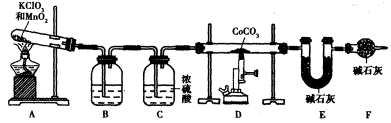
��װ��A�Ƶõ��к�������Cl2����װ��B����ʢ�ŵ��Լ�Ϊ______������ţ���
a��NaHCO3��Һ b��NaOH��Һ c��KMnO4��Һ d������NaCI��Һ
����CoCO3��ȫת��Ϊ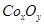 �����Ƶ�E������4��40g��D���ڲ������ʵ�������8��30g����������
�����Ƶ�E������4��40g��D���ڲ������ʵ�������8��30g����������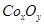 �Ļ�ѧʽΪ____________��
�Ļ�ѧʽΪ____________��
����ȱ��װ��F������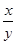 ��ֵ______���ƫ����ƫС������Ӱ�족����
��ֵ______���ƫ����ƫС������Ӱ�족����
(l)���������Һ����2��3.2��pH��5.8��
��3��c����ֹ�����������ܳ�����Co2++2HCO3�� CoCO3��+CO2��+H2O����4��HCl��NH3����5����b����Co2O3����ƫ��
CoCO3��+CO2��+H2O����4��HCl��NH3����5����b����Co2O3����ƫ��
��������������������������֪��������Ϊ�������Һ���ԷϾɵ���������������������������е�⣬����WC����Һ�к����������Ӻ�Co2+������˫��ˮ����������������Ϊ�����ӣ�ͨ�백��������pH��3.2��5.8֮�䣬��������ת��Ϊ���������������˳�ȥ����Һ�к���Co2+������̼�������Һ����̼���ܣ���������CoxOy��(l)���ʱ�Ͼɵ��������������������������������������������Һ����2��ͨ�˰�����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ��pH���Χ��3.2��pH��5.8����3��ʵ����NH4HCO3��Һ�Լ��ԣ��Ʊ�CoCO3ʱ��Ϊ��ֹ�����������ܳ�����ѡ�õļ��Ϸ�ʽ��c����NH4HCO3��Һ�������뵽ʢ����Һ�ķ�Ӧ�����С�����CoCO3�����ӷ���ʽΪCo2++2HCO3�� CoCO3��+CO2��+H2O����4��ʵ���л�õ���ϴ�Ӳ���ֻẬ���Ȼ�����ʣ��ڱ���ʱ�Ȼ�立ֽ�������Ⱦ������HCl��NH3����5�������װ��ͼ��֪��ʵ��ͨ���ⶨװ��E�е����أ�ȷ�����ɵĶ�����̼��������������n��C��=n��Co����֪CoxOy��Co�����ʵ�������������Co��������װ��D���ڲ���������CoxOy���������Ԫ���������ټ�����ԭ�ӵ����ʵ���������ԭ�����ʵ���֮��ȷ����ѧʽ����װ��A���Ƶõ�O2�к���������Cl2��Bװ������ʢ�ŵ�������������Cl2��a������NaHCO3��Һ�������������������ɶ�����̼��Ӱ�������̼�����IJⶨ������b��NaOH��Һ�������������������������䣬��ȷ��c��c��KMnO4��Һ������������������d������NaCl��Һ������������������ѡb����E������4.40g�Ƕ�����̼�����������ʵ���Ϊ0.1mol�����ݻ�ѧʽCoCO3��֪��n��Co��=n��C��=0.1mol��Co������Ϊ0.1mol��59g/mol=5.9g��D���ڲ������ʵ�����8.30g��CoxOy��������CoxOy����Ԫ������Ϊ8.3g-5.9g=2.4g����ԭ�ӵ����ʵ���n��O��=0.15mol��n��Co����n��O��=0.1mol��0.15mol=2��3�����ܵ�������Ļ�ѧʽΪCo2O3����ȱ��Fװ�ã�װ��E�м�ʯ�ҿ������տ�����ˮ������������̼����ɶ�����̼����ƫ����Co������ƫ��x/y��ֵƫ��
CoCO3��+CO2��+H2O����4��ʵ���л�õ���ϴ�Ӳ���ֻẬ���Ȼ�����ʣ��ڱ���ʱ�Ȼ�立ֽ�������Ⱦ������HCl��NH3����5�������װ��ͼ��֪��ʵ��ͨ���ⶨװ��E�е����أ�ȷ�����ɵĶ�����̼��������������n��C��=n��Co����֪CoxOy��Co�����ʵ�������������Co��������װ��D���ڲ���������CoxOy���������Ԫ���������ټ�����ԭ�ӵ����ʵ���������ԭ�����ʵ���֮��ȷ����ѧʽ����װ��A���Ƶõ�O2�к���������Cl2��Bװ������ʢ�ŵ�������������Cl2��a������NaHCO3��Һ�������������������ɶ�����̼��Ӱ�������̼�����IJⶨ������b��NaOH��Һ�������������������������䣬��ȷ��c��c��KMnO4��Һ������������������d������NaCl��Һ������������������ѡb����E������4.40g�Ƕ�����̼�����������ʵ���Ϊ0.1mol�����ݻ�ѧʽCoCO3��֪��n��Co��=n��C��=0.1mol��Co������Ϊ0.1mol��59g/mol=5.9g��D���ڲ������ʵ�����8.30g��CoxOy��������CoxOy����Ԫ������Ϊ8.3g-5.9g=2.4g����ԭ�ӵ����ʵ���n��O��=0.15mol��n��Co����n��O��=0.1mol��0.15mol=2��3�����ܵ�������Ļ�ѧʽΪCo2O3����ȱ��Fװ�ã�װ��E�м�ʯ�ҿ������տ�����ˮ������������̼����ɶ�����̼����ƫ����Co������ƫ��x/y��ֵƫ��
���㣺



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ���������ͼ��ʾ��װ�ý��е绯ѧʵ�飬�������װ��ͼ�ش�������⣺
��1��C������ʲôװ�� ��
��2���缫���Ϸ����缫��ӦʽΪ ��B���з������ܷ�Ӧ����ʽΪ ��
��3����Ӧ����һ��ʱ���A��B��C�����е������ҺŨ�Ȼ����������________��
��4������·����0.2 mol��������ʱ���缫���������仯______g�� �缫���������仯______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
����ѧ�뼼����
�ɻ�ͭ����Ҫ�ɷ���CuFeS2�����ƾ�ͭ�Ĺ�������ʾ��ͼ���£�
��1���ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1000�����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ�����Ҳ���Fe������ת��Ϊ�ͼ�������ù�����������Ҫ��Ӧ�Ļ�ѧ����ʽ�� �� ������¯������¯������Ҫ�ɷ��� ��
��2����ͭ��Cu2S��FeS�����ۺ϶��ɣ���Cu��Ϊ20%��50%��ת¯�н���ͭ���ۼ���ʯӢɰ����1200�����Ҵ���������д�������ͭ�е�Cu2S��������Cu2O�����ɵ�Cu2O��Cu2S��Ӧ�����ɺ�Cu��ԼΪ98.5%�Ĵ�ͭ���ù��̷�����Ӧ�Ļ�ѧ����ʽ�� �� ��
��3����ͭ�ĵ�⾫����ͼ��ʾ��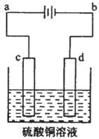
�ڴ�ͭ�ĵ������У���ͭ����ͼ�е缫 ����ͼ�е���ĸ�����ڵ缫d�Ϸ����ĵ缫��ӦΪ ������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
������Ŧ�۵��ΪAg Zn���,���ò�����Ƴ�һ���������Ǻ�������ɵ�СԲ��,���ڿ�������һ�����Ag2O������ʯī��ɵ��������Բ���,������һ�����Zn
Zn���,���ò�����Ƴ�һ���������Ǻ�������ɵ�СԲ��,���ڿ�������һ�����Ag2O������ʯī��ɵ��������Բ���,������һ�����Zn Hg�Ͻ��������Ļ��Բ���,�������ҺΪŨKOH��Һ��д���˵�ص�����������Ӧʽ�Լ�����ܷ�Ӧʽ��
Hg�Ͻ��������Ļ��Բ���,�������ҺΪŨKOH��Һ��д���˵�ص�����������Ӧʽ�Լ�����ܷ�Ӧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС��ͬѧ��̼��Ϊ�缫���CuCl2��Һʱ����������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ��ͬѧ���Ķ����ϲ���������¹��̣�
��.�й����ϣ�ͭ�Ļ�������ɫ��������
| ���� | ��ɫ������ | ���� | ��ɫ������ |
| ������ͭCu(OH)2 | ��ɫ���岻����ˮ | ����ͭ(CuSO4) | ��Һ����ɫ |
| ������ͭ(Cu2O) | ��ɫ���岻����ˮ | �Ȼ�ͭ(CuCl2) | Ũ��Һ����ɫ��ϡ��Һ����ɫ |
| �Ȼ���ͭ(CuCl) | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ظ���������췯�أ��ǻ�ѧʵ�����е�һ����Ҫ�����Լ�����ҵ���Ը���أ�K2CrO4��Ϊԭ�ϣ����õ绯ѧ���Ʊ��ظ���أ�K2Cr2O7�����Ʊ�װ������ͼ��ʾ�������ӽ���Ĥֻ����������������
�Ʊ�ԭ����2CrO42-����ɫ��+2H+ Cr2O72-����ɫ��+H2O
Cr2O72-����ɫ��+H2O
��1��ͨ��������Ҳ���������Ϊ ����缫��Ӧʽ�� ��
��2�����Ʊ������ܷ�Ӧ�����ӷ���ʽ�ɱ�ʾΪ4CrO42-+4H2O="2" Cr2O72- + 4OH- +2H2��+O2������ʵ�鿪ʼʱ�������м���38.8g K2CrO4��t���Ӻ���������K��Cr�����ʵ���֮��Ϊ3:2������Һ��K2CrO4��K2Cr2O7�����ʵ���֮��Ϊ ����ʱ��·��ת�Ƶ��ӵ����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ᡢ��ϩ�ͱ�ϩ��C3H6���Ļ�������������������Ϊa,��̼�����������ǣ� ��
A��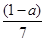 | B��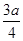 | C��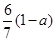 | D��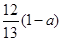 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ijС���ӿ���ҩ��ķ��ӽṹ��ͼ��ʾ������˵����ȷ����(����)
| A��1mol���л��������Ժ�5mol NaOH��Ӧ |
| B�����л����������ӳɡ�ȡ�����к͡���ȥ�ȷ�Ӧ |
| C�����л�����FeCl3��Һ����ɫ������ʹ����KMnO4��Һ��ɫ |
| D��1mol���л�����Ũ��ˮ��Ӧ���������3mol Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����ʵ���������������ȫȼ��ʱ��������������������
| A��C2H4 | B��C2H6 | C��C2H5OH | D��CH3COOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com