
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɡ���ش��������⣺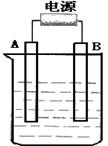
��1����д��B��������ƣ� �缫��Ӧʽ
д�����ʱ��Ӧ�������ӷ���ʽ
��2��������Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g��������Һ��pHΪ ��Ҫʹ������Һ�ָ������ǰ��״̬��������� ��������Ϊ g����������ǰ����Һ��������䣩
��3����ԭ��ҺΪ1L K2SO4��CuSO4�Ļ����Һ����c��SO42-��=" 2.0mol/L" ����ͼװ�õ�⣬���������ռ���22.4L���壨��״����ʱ��ֹͣ��⡣
��ԭ��Һ�е�c(K+)��
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
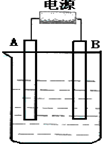 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɡ���ش��������⣺
��1����д��B��������ƣ� �缫��Ӧʽ
д�����ʱ��Ӧ�������ӷ���ʽ
��2��������Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g��������Һ��pHΪ ��Ҫʹ������Һ�ָ������ǰ��״̬��������� ��������Ϊ g����������ǰ����Һ��������䣩
��3����ԭ��ҺΪ1L K2SO4��CuSO4�Ļ����Һ����c��SO42-��= 2.0mol/L ����ͼװ�õ�⣬���������ռ���22.4L���壨��״����ʱ��ֹͣ��⡣
��ԭ��Һ�е�c(K+)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
����ͼ��ʯī���缫�ĵ����У�����500 mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6 g����ش��������⣺

(1)B�缫������Ӧ�ĵ缫��Ӧʽ_________________________��
(2)д�����ʱ��Ӧ�����ӷ���ʽ_________________________________________��
(3)������Һ��H+�����ʵ���Ũ��Ϊ________��Ҫʹ������Һ�ָ������ǰ��״̬���������________��������Ϊ________g��(������ǰ����Һ���������)
(4)ԭ��Һ�п��ܺ��е��������Ϊ�� ��
A CO32- B Cl- C SO42-
���ʵ������������ӣ�д���������裬ʵ�������ʵ�����________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com