
��ѧ��Ӧ��ʵ���ǡ��ɼ��Ķ��Ѻ��¼����γɡ�����һ�������£�һ���Ҵ����Ӷϼ�ֻʧȥ������ԭ�ӣ����ɵ����л������������(д�ṹ��ʽ����һ������)_______________��__________��______________��________________��������ѧϰ����֪ʶ���ṩ���Լ���������ʵ������ijһ��ת��������֤��ת������л�������Լ�����ˮ�Ҵ���������ͭ˿������������Һ������ͭ��Һ���������Թܡ��ԹܼС����ӡ���ͷ�ιܡ��ƾ��ơ����
(1)�Ҵ���ת����
��ʵ�鷽����_____________________________________��
��ʵ��������_____________________________________��
���Ҵ�ת���Ļ�ѧ����ʽ________________________��
(2)�������֤��
�پ������л����������Ҫ���ʵ�ԭ���ŵĽṹʽ��________��������______________��
����֪�ṹ���Ƶ����ʣ��������ƵĻ�ѧ���ʡ�������һԭ����֤���Ҵ�ת���ɵ��л�����������Լ�(д�Լ�����)__________ _��Ȼ���Լ��뱻��֤���л��������ϣ������������ڣ��۲쵽��������______________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
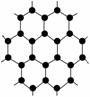 2010��ŵ��������ѧ�����ڱ����״ΰ��������ʯī�Ŀ�ѧ�ҡ�����ʯī��Ϊʯīϩ�����֡�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ����ʯīϩ��������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ�Ӧ��ǰ��ʮ�ֹ��������й���ʯīϩ��������ȷ����(����)
2010��ŵ��������ѧ�����ڱ����״ΰ��������ʯī�Ŀ�ѧ�ҡ�����ʯī��Ϊʯīϩ�����֡�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ����ʯīϩ��������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ�Ӧ��ǰ��ʮ�ֹ��������й���ʯīϩ��������ȷ����(����)
A��ʯīϩ��̼����
B��ʯīϩ��һ���л���
C��ʯīϩ��̼ԭ�ӵĻ��ϼ�Ϊ��3
D��ʯīϩ�ɵ��磬˵�����ǵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�
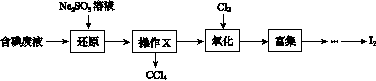
(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
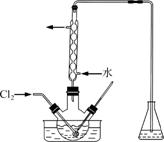
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO ��2IO
��2IO ��2H��===I2��5SO
��2H��===I2��5SO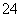 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ������������Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ��ͭ��������ʹ�öԹ��������������涼��������Զ��Ӱ�졣
(1)д��ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ��________________________________________��
(2)Ϊ�˱��������ͽ�Լ��Դ��ͨ������H2O2��ϡ����Ļ����Һ�ܳ��Ͼ�ӡˢ��·���е�ͭ������ʵ��ͭ�Ļ������á�д���ܳ�ͭ�����ӷ���ʽ��__________________________________________��
(3)��ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O��Cu2S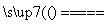 6Cu��SO2���÷�Ӧ�е���������____________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ��ӵ����ʵ���Ϊ________________________________________________________________________mol��
6Cu��SO2���÷�Ӧ�е���������____________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ��ӵ����ʵ���Ϊ________________________________________________________________________mol��
(4)ͭ�ڳ�ʪ�Ŀ������ܷ���������ʴ�����⣬ͭ�����Ҫ�ɷ�ΪCu2(OH)2CO3(��ʽ̼��ͭ)����д�����������и����ĵ缫��Ӧʽ��______________________________________________________________��
(5)�о���ѧϰС���á���ӵ��������ⶨij������CuSO4��5H2O(��������I����Ӧ������������)�ĺ�����ȡa g�������100 mL��Һ��ÿ��ȡ25.00 mL����Һ���μ�KI��Һ���а�ɫ�⻯��������ɣ�д���÷�Ӧ�����ӷ���ʽ��____________________________�������μ�KI��Һ���������ٲ�������Һ�е�I2����������Ʊ���Һ�ζ���������Ӧ�Ļ�ѧ����ʽΪI2��2Na2S2O3===2NaI��Na2S4O6��ƽ������c mol/L Na2S2O3��Һ�����ΪV mL����������CuSO4��5H2O����������Ϊ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ܷ�����ȥ��Ӧ����(����)
A��1���������NaOH����Һ����
B����������NaOH��ˮ��Һ����
C���Ҵ���ŨH2SO4������140��
D���Ҵ���ŨH2SO4������170��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ���ܴ����������
A�����뱽������ɫ����Һ��K����NH4����Cl����I��
B�������̪�ʺ�ɫ����Һ��SO42����K����Cl����HSO3��
C�� ��1��10��12����Һ�� K����AlO2����CO32����Na��
��1��10��12����Һ�� K����AlO2����CO32����Na��
D��pH��2����Һ��NO3����Fe2����Na����Al3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ����(����)
A����������������Fe2O3��6H��===2Fe3����3H2O
B���������ʵ�����KHCO3��Ba(OH)2��Һ��ϣ�
HCO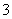 ��Ba2����OH��===BaCO3����H2O
��Ba2����OH��===BaCO3����H2O
C�����Ȼ�����Һ�м��������ˮ��Al3����4NH3��H2O===AlO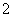 ��4NH
��4NH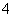 ��2H2O
��2H2O
D������ˮ��Һ�ʼ��ԣ�S2����H2O HS����OH��
HS����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��ӷ����������(����)
| ѡ�� | ���ᴿ������ | ���� | �����Լ� | ���ӷ��� |
| A. | CO(g) | CO2(g) | NaOH��Һ�� ŨH2SO4 | ϴ�� |
| B. | NH4Cl(aq) | Fe3��(aq) | NaOH��Һ | ���� |
| C. | Cl2(g) | HCl(g) | ����ʳ��ˮ�� ŨH2SO4 | ϴ�� |
| D. | Na2CO3(s) | NaHCO3(s) | �� | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com