
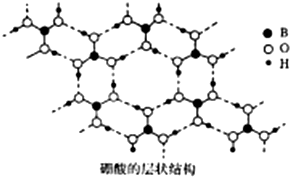
 ��B�����仯�����ڻ�ѧ������Ҫ�ĵ�λ����ش��������⣺
��B�����仯�����ڻ�ѧ������Ҫ�ĵ�λ����ش��������⣺ [B��OH��4]-+H+��
[B��OH��4]-+H+������ ��1��Ga��Bͬ���壬���ڵ������ڢ�A�壬���������Ϊ31����Ϻ�������Ų�������д��ͬ���ڴ����ҵ�һ�����������ǵ�IIA�͵�IIIA�塢��VA�͵�VIA������
��2������ͼ��֪��Bԭ���γ�3��B-O�Ҽ���û�йµ��Ӷԣ��ݴ��ж�Bԭ���ӻ���ʽ��
�������ᾧ��ṹ��֪�����ڷ�����O��B��H֮���γɹ��ۼ������Ӽ�H��O֮���γ������
�ۼ����ƻ����������֮�����������������ˮ����֮���γ������
��������һԪ���ᣬ��ˮ�е���ʱ��������ˮ�������OH-�������ԣ�����������[B��OH��4]-��H+��
��3������������Ľṹ�����嶼���ƽ��ʯ����ÿ��Bԭ���γ�4�����ۼ������ɵ�������״�ṹ�����ݹ��ۼ���Ŀ�ж��ӻ����ͣ�
��� �⣺��1��Ga��Bͬ���壬���ڵ������ڢ�A�壬���������Ϊ5+8+18=31�����ݹ���ԭ��֪���̬ԭ�Ӻ�����ӷֲ�ʽΪ1s22s22p63s23p63d104s24p1��ͬ���ڴ����ҵ�һ�����������ǵ�IIA�͵�IIIA�塢��VA�͵�VIA�������ʵ�һ�����ܣ�C��Be��B��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1�� C��Be��B��
��2������ͼ��֪��Bԭ���γ�3��B-O�Ҽ���û�йµ��Ӷԣ�Bԭ���ӻ������ĿΪ3��Bԭ�Ӳ�ȡsp2�ӻ���ʽ���ʴ�Ϊ��sp2��
�������ᾧ��ṹ��֪�����ڷ�����O��B��H֮���γɹ��ۼ������Ӽ�H��O֮���γ�����������֮��Ϊ���»�����
�ʴ�Ϊ�����ۼ��������
�ۼ����ƻ����������֮�����������������ˮ����֮���γ����������ʱ��������ܽ������
�ʴ�Ϊ�������ƻ����������֮�����������������ˮ����֮���γ������
��������һԪ���ᣬ��ˮ�е���ʱ��������ˮ�������OH-�������ԣ�����������[B��OH��4]-��H+�����뷽��ʽΪ��H3BO3+H2O?[B��OH��4]-+H+��
�ʴ�Ϊ��H3BO3+H2O?[B��OH��4]-+H+��
��3������������Ľṹ�����嶼���ƽ��ʯ�����ʯ��ÿ��Cԭ��������4��̼ԭ���γ�������ṹ����������Ϊ������״�ṹ����ÿ��Bԭ���γ�4�����ۼ�����Bԭ�ӵ��ӻ�����Ϊsp3��ÿ��Bԭ���γ�4�����ۼ�����1mol������������B-N�����ʵ���Ϊ4mol��
�ʴ�Ϊ��sp3��4mol��
���� �����Ƕ����ʽṹ�Ŀ��飬��Ŀ�漰�����Ų�ʽ����һ�����ܡ��ӻ����۵�Ӧ������ͷ��Ӽ��������ȣ��漰֪ʶ��϶࣬��Ҫѧ���߱���ʵ�Ļ�������ʵǨ�������������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ֲ��ͨ�����������տ����еĵ��ǻ�ѧ�仯 | |
| B�� | ��ʯȼ��ȼ��ͨ�����ͷų����������� | |
| C�� | ����β�����ŷŵĵ�����������������̬��ת������ | |
| D�� | ֲ��ո�ȼ��ʱ�ų���������������˵���ѭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
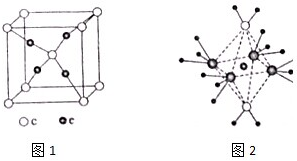 ���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ��b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c�����������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӣ��ش��������⣺
���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ��b�ļ۵��Ӳ��е�δ�ɶԵ�����3����c�����������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӣ��ش��������⣺ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �������� | ԭ�Ӿ��� | ���Ӿ��� | ���Ӿ��� |
| A�� | ������ | ���� | ������ |
| B�� | ������ | ̼����� | ˮ�� |
| C�� | ���ʯ | �ռ� | �� |
| D�� | �� | ���� | ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȫ�� | B�� | ���ܢޢ����� | C�� | �ۢܢݢ� | D�� | �ڢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ʾ�����й����л��� I��˵����ȷ����CD��
��ʾ�����й����л��� I��˵����ȷ����CD��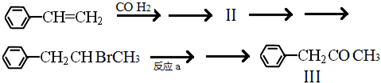
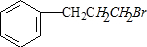 ��
�� ��
�� +NaOH$��_{��}^{H_{2}O}$
+NaOH$��_{��}^{H_{2}O}$ +NaBr��
+NaBr���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�������ӻ�����X2Y | |
| B�� | ֻ���ǹ��ۻ�����X2Y2 | |
| C�� | �ȿ��������ӻ�����Ҳ�����ǹ��ۻ����� | |
| D�� | �γɵĻ�����������X2Y����X2Y2 ԭ�Ӷ��ﵽ��8�����ȶ��ṹ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com