
 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�
 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ___________________��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ___________________�� Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ� ���úϳ�·������ͼ��ʾ����ע����Ӧ��������
���úϳ�·������ͼ��ʾ����ע����Ӧ��������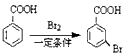 ���������
��������� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �Ҵ� |
| �� |
| �Ҵ� |
| �� |
 �����
��д���� Ϊԭ���Ʊ�
Ϊԭ���Ʊ�  �ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£� ��
��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�


 �г����Ѽ���-S-���⣬�����еĹ���������Ϊ
�г����Ѽ���-S-���⣬�����еĹ���������Ϊ| �� |
| �� |
 һ���������γɾۺ���Ľṹ��ʽΪ
һ���������γɾۺ���Ľṹ��ʽΪ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�


 �г����Ѽ���-S-���⣬�����еĹ���������Ϊ
�г����Ѽ���-S-���⣬�����еĹ���������Ϊ| �� |
| �� |
 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�  ���úϳ�·������ͼ��ʾ����ע����Ӧ��������
���úϳ�·������ͼ��ʾ����ע����Ӧ�������� ����������ںϳɹ��������Լ���ѡ���������ñ����������Ϣ��
����������ںϳɹ��������Լ���ѡ���������ñ����������Ϣ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ƽ�����ھ��������ƣ���ṹΪ��
( )2��
)2�� �����ĺϳ�·�����£�
�����ĺϳ�·�����£�

��֪������Ӧ��Ϊȡ����Ӧ������A��ϵͳ����Ϊ1,4������D2�D��ϩ��
��ش��������⣺
��д������ʽΪC13H9NO4S�����ʵĽṹ��ʽ ��
������ �г����Ѽ�����S�����⣬�����еĹ���������Ϊ ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ ��
�Ƿ�Ӧ�۵������� ��Ӧ����Ӧ�ݵ�Ŀ���� ��
��д����Ӧ�ܵĻ�ѧ����ʽ ��
������B��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹���� �֡�
����֪����SH�������룭OH���ơ�
���� һ���������γɾۺ���Ľṹ��ʽΪ ��
һ���������γɾۺ���Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ƽ�����ھ��������ƣ���ṹΪ��
()2��
�����ĺϳ�·�����£�
��ش��������⣺
��д������ʽΪC13H9NO4S�����ʵĽṹ��ʽ ��
�������г����Ѽ�����S�����⣬�����еĹ���������Ϊ ��
�Ƿ�Ӧ�ߵ������� ��Ӧ����Ӧ�ݵ�Ŀ���� ��
��д����Ӧ�ܵĻ�ѧ����ʽ ��
����Ϊԭ�Ϻϳ�
���úϳ�·������ͼ��ʾ����ע����Ӧ��������
��ʾ���� ��֪�������ϵ��Ȼ�Ϊ��λ��λ������ ���������
�ںϳɹ��������Լ���ѡ���������ñ����������Ϣ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com