
| 1000�Ѧ� |
| M |
| n |
| V |
| 1000��1.84��98% |
| 98 |
| n |
| V |


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
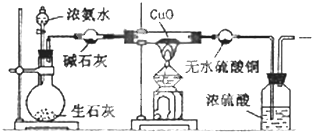
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�B�ĵ��ʷ����������Թ��õ��ӣ�C�����������Ǵ�����������3����A��Dͬ���壬Eԭ�ӵ�������Cԭ�ӵ�������5������ش��������⣺
ԭ���������������A��B��C��D��E���ֶ�����Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�B�ĵ��ʷ����������Թ��õ��ӣ�C�����������Ǵ�����������3����A��Dͬ���壬Eԭ�ӵ�������Cԭ�ӵ�������5������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
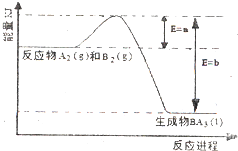
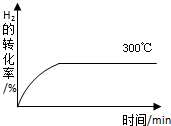 �״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ��һ��ɲ������·�Ӧ���ϳɼ״���2H2��g��+CO��g���TCH3OH��g�� �±�Ϊ��ͬ�¶��µ�ƽ�ⳣ����K��
�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ��һ��ɲ������·�Ӧ���ϳɼ״���2H2��g��+CO��g���TCH3OH��g�� �±�Ϊ��ͬ�¶��µ�ƽ�ⳣ����K��| �¶� | 250�� | 300�� | 350�� |
| K | 2.041 | 0.270 | 0.012 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������Ƶ���ˮ�ε���ɫ��ʯ����ֽ�ϣ���ֽ�ȱ�죬˵����ˮ�к���H+ |
| B������ˮ�еμ������ữ��AgNO3��Һ��������ɫ������˵����ˮ�к���Cl- |
| C������ˮ�м���NaHCO3��ĩ�������ݲ�����˵����ˮ�к���H+ |
| D����ˮ��ʹ��ɫ������ɫ��˵����ˮ�к���Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com