
| ������ | Ag+��Na+ |
| ������ | NO3-��SO42-��Cl- |
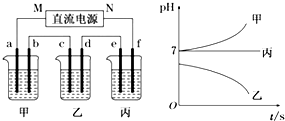
���� ��1����������C�缫����������54�ˣ�˵��C�缫������������C�缫�ĵ�ԴM�缫Ϊ������NΪ������
��2������C�缫���������ӣ������������ʣ�������װ���к��������ӣ��ֵ����װ���е������Һ����Һ��pHֵ��С�������������������������ӣ��������ӵķŵ�˳���жϵ���ʣ����ʱ����װ������Һ��pHֵ����˵�������������ӷŵ磬�����Ϸŵ�����������������ǿ�����ӷŵ磬�ݴ��жϵ���ʣ���װ�õ������Һ��NaCl��Һ���ҵ������Һ��AgNO3��Һ����Ϊ�����ƻ��������Һ�����ˮf�缫�ϲ���������ϵ�·�ϵ����غ����f�缫�ϲ��������������
��3���ҵ������Һ��AgNO3��Һ������������������ᡢAg��������
��4����װ�õ������Һ��NaCl��Һ����ⷽ��ʽ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2�������ݵ����غ����������������Ƶ�Ũ�ȣ��ټ���pH��
��5����Ϊ�����ƻ��������Һ�����ˮ������ˮ�������仯������
��� �⣺��1����ͨ��Դ������һ��ʱ��������C�缫����������27�ˣ�˵��C�缫���������������������ĵ缫M��ԭ��صĸ�����NΪ������
�ʴ�Ϊ������
��2����ͼ��֪�����ʱ����װ������Һ��pHֵ����˵���������Ƿŵ������������������ӵ����ӷŵ磬���ݱ���֪��Ϊ�����ӣ������Ӻ������������ɳ��������Լ�װ�õ������Һ��NaCl��Һ����ΪC�缫���������ӣ������������ʣ�������װ���к��������ӣ���ͼ2��֪�����ʱ��װ������pHֵ��С��˵���������������ӷŵ磬��Һ�к��е��������Ǻ���������ӣ��õ������Һ��AgNO3��Һ������C�缫����������54�ˣ�����Ag+e-=Ag������ת�Ƶ�����$\frac{54g}{108g/mol}$=0.5mol������pHֵ���䣬˵��Ϊ���ˮ�ͣ�Ϊ�����ƻ��������Һ���缫fΪ��������ӦʽΪ4OH--4e-=2H2O+O2�����������ɱ�״���µ����Ϊ��$\frac{0.5mol}{5}$��22.4L/mol=2.8L��
�ʴ�Ϊ��2.8��
��3���ҵ������Һ��AgNO3��Һ������������������ᡢAg�����������ⷽ��ʽΪ��4AgNO3+2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4HNO3��
�ʴ�Ϊ��4AgNO3+2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4HNO3��
��4����װ�õ������Һ��NaCl��Һ����ⷽ��ʽ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2����������n��NaOH��=0.5mol������c��NaOH��=$\frac{0.5mol}{5L}$=0.1mol/L������Һ��pH=13��
�ʴ�Ϊ��13��
��5����Ϊ�����ƻ��������Һ��������ˮ��2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2����ת��0.5mol���ӣ�����ˮΪ0.25mol����ˮ������Ϊ0.25mol��18g/mol=5.4g��
�ʴ�Ϊ��5.4g��H2O��
���� ���⿼����ԭ��غ͵���ԭ������ȷ��C�缫�������ı仯������˼�ǽⱾ��Ĺؼ�������ԭ��غ͵���ʸ��缫�Ϸ�����Ӧ�����ͼ��ɽ���⣬��Ŀ�Ѷ��еȣ�


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2���к�ǿ�������ԣ��ڻ�ѧ��Ӧ��ֻ���������� | |
| B�� | Cl-��ClΪ��ͬ�ĺ��أ��в�ͬ�Ļ�ѧ���� | |
| C�� | ʵ�����Ʊ�Cl2�������ű���ʳ��ˮ�������ռ� | |
| D�� | ��ⱥ��ʳ��ˮ������ʱ�����Դ����������ʯī���Ϸ���������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
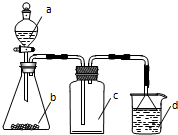 ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã����ô�װ�úͱ����ṩ��������������ʵ����ǣ�������
ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã����ô�װ�úͱ����ṩ��������������ʵ����ǣ�������| ѡ�� | a������ | b������ | c�е����� | d������ |
| A | Ũ��ˮ | CaO | NH3 | H2O |
| B | ϡ���� | Cu | NO | H2O |
| C | ���� | Na2CO3 | CO2 | NaOH��Һ |
| D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ������ | ���� |
| A | ������Al3+����Һ�У�K+��Na+��NO3-��CO32- | ���ܴ������棬����Al2��CO3��3�������� |
| B | ������Fe3+����Һ�У�K+��Mg2+��I-��NO3- | ���ܴ������棬��2Fe3++2I-=2Fe2++I2 |
| C | ��ˮ�����c��H+��=1��10-14mol/L����Һ�У� Ca2+��NO3-��HCO3-��Cl- | ���ܴ������棬����Һ�����ԣ���HCO3-��Ӧ����CO2���� |
| D | ʹ��̪������Һ�У� Na+��K+��SO32-��S2- | ���ܴ������棬��SO32-��S2-��Ӧ��������ɫ��S���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������̼����ƺ��Ȼ��Ƶ�ˮ������Ӳˮ | |
| B�� | �����о�ˮ���ã���������������Ӳˮ������ʹӲˮ���� | |
| C�� | �����ӽ���������Ӳˮ��ֻ��������ʱӲˮ��������������Ӳˮ | |
| D�� | ֻ������ʱӲ�ȵ�ˮ��������кɱ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��NO3-��Al3+��K+ | B�� | Na+��NO3-��S2-��K+ | ||
| C�� | MnO4-��SO32-��Na+��K+ | D�� | HCO3-��SO42-��Na+��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| X | ||||
| Z | W |
| A�� | X������ɵĻ���������п��ܺ��м��Թ��ۼ��ͷǼ��Թ��ۼ� | |
| B�� | ��ҵ���õ��Y��W��ɵĻ��������Ʊ�Y | |
| C�� | Z��W��Ԫ�ص���̬�⻯���У�W����̬�⻯����ȶ� | |
| D�� | X��Y��Z��WԪ������������Ӧ��ˮ������������ǿ����HWO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Ըı�ƽ��״̬ | B�� | �ﵽƽ��ʱ�����淴Ӧ������� | ||
| C�� | ƽ��ʱ����ֵĺ���Ҳ�ڲ��ϱ仯 | D�� | �÷�Ӧ��������ƽ���Ƕ�̬ƽ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com