
�����жϣ���ȷ�Ļ����̡�������Ļ�������
(1)������ƿ����Һʱ������ˮ�����̶��ߣ������õι���������Һ��(����)
(2014·�¿α�ȫ������12D)
(2)������Һ����ʱ����������ƿ�̶Ȼ�ʹ��ҺŨ��ƫ��(����)
(2014·���ȫ������6C)
(3)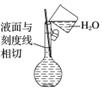 ������Һ(����)
������Һ(����)
(2014·ɽ�����ۣ�10A)
(4)��ΪKNO3���ܽ�ȴ����Կ����ؽᾧ����ȥKNO3�л��е�NaCl(����)
(2014·�㶫���ۣ�9A)
(5)��ΪCa(OH)2���Ƴɳ���ʯ��ˮ�����Կ�����2.0 mol·L��1��Ca(OH)2��Һ(����)
(2014·�㶫���ۣ�9D)
(6)����0.100 0 mol·L��1�Ȼ�����Һʱ����Һ��ת�Ƶ�����ƿ�����ò���������(����)
(2013·�������ۣ�10C)
(7)������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС(����)
(2013·������ۣ�4B)
(8)�ù���NaCl����0.5 mol·L��1����Һ�����õ������У��ձ�������������ͷ�ιܡ�����ƿ(����)
(2013·ɽ�����ۣ�11C)
(9)�� ����100 mL 0.100 0 mol·L��1 K2Cr2O7��Һ(����)
����100 mL 0.100 0 mol·L��1 K2Cr2O7��Һ(����)
(2012·������ۣ�4D)
(10)��50 mL��Ͳ������0.100 0 mol·L��1̼������Һ(����)
(2012·�������ۣ�6C)
(11)��100 mL����ƿ���ձ�������������ͷ�ιܺ�pH��1����������100 mL pH��2������(����)
(2012·�������ۣ�10B�ı�)
(12)����Ͳ��ȡ20 mL 0.500 0 mol·L��1 H2SO4��Һ���ձ��У���ˮ80 mL�����Ƴ�0.100 0 mol·L��1 H2SO4��Һ(����)
(2011·���գ�7B)
(13)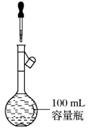 ���ݲ���(����)
���ݲ���(����)
(2010·�������ۣ�8B)
(14)������Һʱ������ˮ��������ƿ�̶ȣ�Ӧ�ý�ͷ�ιܽ�������Һ����(����)
(2010·ɽ�����ۣ�14B)
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�ѡ���������Ӧ������ͬһ��Ӧ���͵��ǣ� ��
| ѡ�� | ��Ӧ�� | ��Ӧ�� |
| A | �ڹ��������£���������������Ӧ��ȡCH3CH2Br | ����ϩͨ��������Ȼ�̼��Һ����ȡ1��2-�������� |
| B | �����������������£�����������Ӧ���ɻ����� | ��һ�������£���ϩͨ�����������ֱ����ˮ��Ӧ�����Ҵ� |
| C | �����������������£��Ҵ�������е�������Ӧ������ȩ | ��һ�������£�������ϩ(CH2��CHCl)�ϳɾ�����ϩ |
| D | ������������Ĵ���������ˮ��Ӧ����������Ҵ� | ��һ�������£�Һֲ̬������������Ӧ������֬�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA���������ӵ�����������������ȷ����(����)
A����״���£�4.0 g CH4�к��й��ۼ�����ĿΪNA
B�����³�ѹ�£�6.4 g�����ͳ����к��еķ�������Ϊ0.2NA
C�����³�ѹ�£�22.4 L NH3Լ����NA��NH3����
D��һ��������6.4 g SO2������������Ӧ����SO3��ת�Ƶ�����Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������Ϊ98%��Ũ�����������������Ϊ18%��ϡ��������500 g������������Ϊ28%�����ᣬ��ҪŨ�����ϡ����������ֱ�Ϊ(����)
A��62.5 g��437.5 g B��71.4 g��428.6 g
C��437.5 g��62.5 g D��428.6 g��71.4 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�á�ƫ����ƫС������Ӱ�족���
(1)����450 mL 0.1 mol·L��1��NaOH��Һ����������ƽ��ȡNaOH����1.8 g________��
(2)����500 mL 0.1 mol·L��1������ͭ��Һ����������ƽ��ȡ����8.0 g________��
(3)����NaOH��Һʱ����ƽ�����������Ϸ�����������ȵ�ֽƬ��������������ȷ________��
(4)����һ�����ʵ���Ũ�ȵ�NaOH��Һ�����������4.4 g������ʱ������õߵ�________��
(5)����Ͳ��ȡŨ����ʱ�����Ӷ���________��
(6)����NaOH��Һʱ���������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в�����________��
(7)����ʱ����ˮ�����̶��ߣ��ý�ͷ�ι���ȡ�����Һ�����̶���________��
(8)����ҡ�Ⱥ���Һ���½���������ˮ���̶���________________________________________________________________________��
(9)����ʱ���ӿ̶���________��
(10)����ҡ�Ⱥ�������Һ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ����ʵ���������ȫ��ȷ����(����)
A��������ƿ���ȼ���һ�������ˮ���ټ���Ũ��������ȷŨ�ȵ�ϡ����
B����Ũ��������1��1(�����)��ϡ����(Լ6 mol·L��1)ͨ����Ҫ������ƿ������
C������NH4Fe(SO4)2����Һʱ������һ����H2SO4�Է�ˮ��
D����pH��1����������100 mL pH��2����������ȫ������������100 mL����ƿ���ձ���
����������ͷ�ι�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��
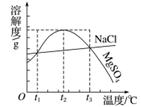
ͼ��NaCl��MgSO4���ܽ�����ߡ�����˵����ȷ����(����)
A��ֻ����t1 ��ʱ��NaCl��MgSO4���ܽ�Ȳ����
B��t1��t2 �棬MgSO4���ܽ�����¶����߶���С
C����t2 ��ʱ��MgSO4������Һ�����������������
D����MgSO4������Һ���¶ȴ�t3 �潵��t2 ��ʱ��û�о�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���ȷ����(����)
A����һ����̬����ӵ�ԭ���У������������˶�״̬��ȫ��ͬ�ĵ���
B����һ����̬����ӵ�ԭ���У�������������������ȫ��ͬ�ĵ���
C����һ����̬����ӵ�ԭ���У�M���ϵĵ��������϶���L���ϵĵ���������
D�����ijһ��̬3p�ܼ��Ͻ���2�����ӣ��������������Ȼ�෴
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D����Ԫ�أ�����AԪ�غ�BԪ�ص�ԭ�Ӷ���1��δ�ɶԵ��ӣ�A����B����һ�����Ӳ㣬Bԭ�ӵ�һ�����Ӻ�3p���ȫ����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ40%���������������������������R����A��D��Ԫ���γɵ����ӻ��������A��D������֮��Ϊ2��1����ش��������⣺
(1)A���ʡ�B���ʡ�������R���۵��С˳��Ϊ���е�________(�����)��
��A����>B����>R ��R>A����>B����
��B����>R>A���� ��A����>R>B����
(2)��CB3������CԪ��ԭ�ӵ�ԭ�ӹ����������______�ӻ��������ʱ�ľ�������Ϊ________��
(3)д��Dԭ�ӵĺ�������Ų�ʽ��______________________________��C���⻯���D���⻯����ˮ�е��ܽ�ȴ�ö�Ŀ���ԭ����__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com