
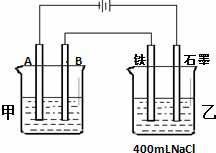
 ��ͼ��ʾΪ������ļס����������أ��Իش�
��ͼ��ʾΪ������ļס����������أ��Իش����� ���ݵ�Դ�����������ж�A��B��Fe��ʯī�ֱ�Ϊ���ص��������������������������������Ϸ�����ԭ��Ӧ���������Ϸ���������Ӧ���ҳط�Ӧ��������2H2O+2e-�TH2��+20H-��������2Cl--2e-�TCl2��������Ϊ�õ��ԭ������Ƭ������װ�ã�������ӦΪ��Ag++e-=Ag��������ӦΪ��Ag-e-=Ag+���������ҺӦѡ��Ʋ������ͬ�������ӵĿ���������Һ�����ݵ缫��Ӧ�Լ�������������ת�Ƶ�����Ŀ��ȼ��㣮
��� �⣺���ݵ�Դ�����������ж�A��B��Fe��C�ֱ�Ϊ���ص��������������������������������Ϸ�����ԭ��Ӧ���������Ϸ���������Ӧ��
��1������Ƭ�϶���ʱ���Ʋ������Ϊ���ص������������ϵĵ缫��ӦʽΪAg-e-=Ag+���Ƽ�������Ϊ���ص������������ϵĵ缫��ӦʽAg++e-=Ag���������Һ���жƲ�������ӣ�ӦΪ��������������Һ��
�ʴ�Ϊ������AgNO3��Ag-e-=Ag+��
��2���ҳ����ǵ�ⱥ��ʳ��ˮ����Һ�е��������������õ����ӷ�����ԭ��Ӧ�����������ƻ���ˮ�ĵ���ƽ�⣬����������Ũ����������̪��죻��Fe�缫������죻
�ʴ�Ϊ������
��3���ײ�������ӦΪAg++e-=Ag����������43.2g��ӦΪ����������n=$\frac{43.2g}{108g/mol}$=0.4 mol��
ת�Ƶĵ���Ϊ0.04mol���������ش�����ת�Ƶĵ�����Ŀ��ȣ��Ҳ�������ӦΪ2Cl--2e-�TCl2����ת�Ƶĵ���Ϊ0.4molʱ����������������������ʵ���Ϊ0.2mol���ų������ڱ�״���µ����Ϊ0.2mol��22.4L/mol=4.48L=4480mL��
�ҳصĵ�ط�ӦʽΪ2NaCl+2H2O=Cl2��+H2��+2NaOH�����������ɵ�����Ϊ�������ƣ����������Ƶ����ʵ���Ϊx����
2NaCl+2H2O=Cl2��+2NaOH+H2�� ת�Ƶ���
2mol 2mol
x 0.4mol
����x=0.4mol
�������Ƶ����ʵ���Ũ��C=$\frac{0.4mol}{0.4L}$=1mol/L��c��H+ ��=$\frac{Kw}{C��O{H}^{-}��}$=$\frac{1��1{0}^{-14}}{1}$mol/L=1��10-14mol/L����pH=14��
�ʴ�Ϊ��4480�� 1mol/L��14��
���� ���⿼����صĹ���ԭ����Ϊ�߿��������ͺ�Ƶ���㣬������ѧ���ķ��������ͼ��������Ŀ��飬��Ŀ�ѶȲ�����ע��缫��Ӧʽ����д�Լ���������ת�Ƶ�����ȵ��ص㣮


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuH��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ2CuH+2HCl�TCuCl2+2H2��+Cu | |
| B�� | CuH������������ȼ�յĻ�ѧ����ʽΪ2CuH+Cl2�T2Cu+2HCl | |
| C�� | CuH��ϡ���ᷴӦ�Ļ�ѧ����ʽΪCuH+3HNO3�TCu��NO3��2+NO��+2H2O | |
| D�� | CuH���ȷֽ�Ļ�ѧ����ʽΪ2CuH�T2Cu+H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������п��Һ��������п��Һ����ʵ�飬�ܹ۲쵽ͬ�������� | |
| B�� | �����ܽ�������������[Zn��NH3��4]2+ | |
| C�� | ��Ӧ����Һ�в������κγ��������Է�Ӧǰ��Zn2+��Ũ�Ȳ��� | |
| D�� | ��[Zn��NH3��4]2+�����У�NH3�����¶Ե��ӣ�Zn2+�ṩ�չ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
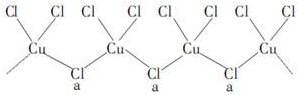
 ������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ҺA�е�������ΪFe2+��Fe3+��H+ | B�� | ��Ʒ��FeԪ�ص�����Ϊ2.24 g | ||
| C�� | ��Ʒ��CuO������Ϊ4.0 g | D�� | V=896 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2��NaClO | B�� | Fe2O3��Fe��OH��3 | C�� | Na��Na2SO4 | D�� | Cu��CuCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol Cl2�μӷ�Ӧת�Ƶ�����һ��Ϊ2NA | |
| B�� | ���³�ѹ�£�NA��CH4����ռ�е����ԼΪ22.4L | |
| C�� | ���³�ѹ�£�1mol Na2O2��������Ϊ2NA�� | |
| D�� | ��״���£�11.2L16O2��11.2L18O2������NA����ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com