



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������и����������¿�����ѧ�Ծ� ���ͣ������
��18�֣���ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
I��Ħ���������������Ķ��Լ���
ȡ����������Ʒ����ˮ��ֽ��衢���ˡ�
��1���������м������ NaOH��Һ�����ˡ�����������NaOH��Һ��Ӧ�����ӷ���ʽ��________________________________________________________________________________��
��2������1��������Һ����ͨ�����������̼���ټ������ϡ���ᡣ�۲쵽��������_____________________________________ ______________________________________��
II��������Ʒ��̼��ƵĶ����ⶨ
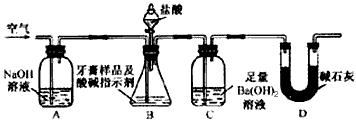
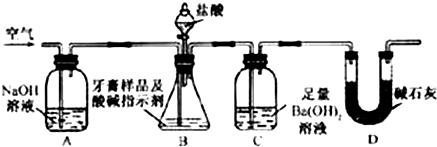
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��3��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У�______________________________________________________________________��
��4��Dװ�õ�������__________________________________________________��
��5��C�з�Ӧ����BaCO3�Ļ�ѧ����ʽ��________________________________________��
��6�����и����ʩ�У�������߲ⶨȷ�ȵ���__________�����ţ���
a���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
b���μ�����˹���
c����A-B֮������ʢ��Ũ�����ϴ��װ��
d����B-C֮������ʢ�б���̼��������Һ��ϴ��װ��
��7��ʵ����ȷ��ȡ8.00 g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94 g������Ʒ��̼��Ƶ���������Ϊ__________��
��8��������Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ��̼��Ƶ�����������ʵ��֤�����˷����ⶨ�Ľ������ƫ�ߣ�ԭ����____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ��һ12���¿���ѧ�Ծ� ���ͣ������
10�֣�ij��ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京����������̽����
������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
��.Ħ���������������Ķ��Լ���
ȡ����������Ʒ����ˮ�ɷֽ��衢���ˡ�
��1���������м������NaOH��Һ�����ˡ�����������NaOH��Һ��Ӧ�����ӷ���ʽ��__________________________ ___ ______��
��2������1��������Һ����ͨ�����������̼���ټ������ϡ���ᡣ�۲쵽��������______________________ ___ ______��
��.������Ʒ��̼��ƵĶ����ⶨ
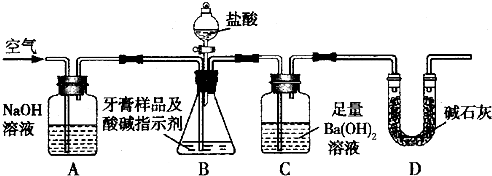
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������
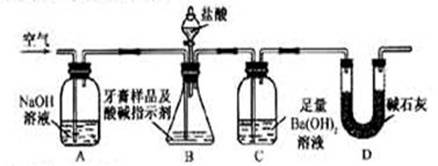
����ʵ����̻ش��������⣺
��3��ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬���У�____________________ _____________��
��4��C�з�Ӧ����BaCO3�Ļ�ѧ����ʽ��_______________________ _________��
��5��ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g ������Ʒ��̼��Ƶ���������Ϊ_________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com