
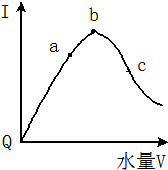
 �������£��������ˮϡ�����У���Һ��������I�����ˮ����V��ʾ������ı仯������ͼ��ʾ����ش�
�������£��������ˮϡ�����У���Һ��������I�����ˮ����V��ʾ������ı仯������ͼ��ʾ����ش����� ��1����Һ�ĵ�����������Ũ���йأ�����Ũ��Խ������Խǿ��
��2����������Խǿ������Ũ��Խ��������Ũ��Խ��pHԽС����ҺԽϡ��Խ�ٽ�������룻
��3��Ҫʹ���������Ũ�������Բ��ü��ȡ����뺬�д�������ӵ����ʡ�����������ӷ�Ӧ�����ʣ�
��� �⣺��1����Һ�ĵ�����������Ũ���йأ�����Ũ��Խ������Խǿ����������û�������ƶ������ӣ����Ա�������磬
�ʴ�Ϊ����Ϊ������δ���룬�������ƶ������ӣ�
��2����������Խǿ������Ũ��Խ��������Ũ��Խ����a��b��c������Һ��������Ũ����С�����˳��ΪΪc��a��b����ҺԽϡ��Խ�ٽ�������룬����Һ�������ӵ����ʵ���Խ����̶�Խ�ʴ�Ϊ��c��a��b��a��b��c��
��3����NaOH���壬�������ƺ������ӷ�Ӧ�ٽ�������룬���Դ��������Ũ�����ӹ���CH3COONa�������ƴ�����룬�������Ƶ�����Ĵ�������Ӵ������ƴ��������Ĵ�������ӣ����Դ��������Ũ��������п�����������ӷ�Ӧ���ٽ�������룬���Դ��������Ũ������MgO���壬�������ӷ�Ӧ���ٽ�������룬��Na2CO3�����������ӷ�Ӧ���ٽ�������룬
�ʴ�Ϊ��������������ι��壬���ý��������������̼���εȣ�
���� �����ۺϿ������ʵĵ��룬������ѧ���ķ��������Ŀ��飬Ϊ�߿��������ͺ�Ƶ���㣬��Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1
�̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | BeCl2Ϊ���ۻ����� | |
| B�� | At2Ϊ��ɫ���壬HAt���ȶ���AgAt�й���ǿ����������ˮҲ������ϡ�� | |
| C�� | ���ᱵ��������ˮ�İ�ɫ���� | |
| D�� | ����������ɫ���ж�����H2S�ȶ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ������ | ������ |
| A | ����ˮ��ʱ�������������ԵĽ������� | ������������ˮ���� |
| B | Fe3+�������� | FeCl3��Һ�������ܽ���վɵ�·�� �е�ͭ |
| C | NH4Cl���ȷֽ� | ���ȿɽ�Ca��OH��2��NH4Cl����������� |
| D | SO2���������� | SO2������Ư��ֽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | ⑬ | |||||||
| 2 | �� | ⑭ | �� | |||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ⑪ | ⑫ |
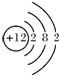 ����
����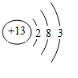 ����
����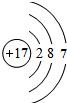 ��
��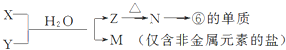
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

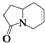 ���ǣ�������
���ǣ�������| A�� | 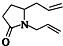 | B�� | 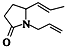 | C�� | 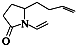 | D�� | 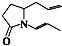 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��$\underset{\stackrel{16}{\;}}{8}{O}_{2}$��Ϊͬλ�� | B�� | ������������ͬ�Ļ�ѧ���� | ||
| C�� | ��������Ϊͬ�������� | D�� | ��ͬ����������������ͬ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com