

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)ͼA��ʾ��Һ��_____________________________���ӵı仯���ߣ�˵�������½���ԭ��_____________________________________________________________________��
(2)����Һ���е�����ʵ�飬��ͨ��Ļ�������������V1ʱ��������Һ������������ǿ����Ҫ��������___________��
(3)��n=0.02 molʱ������ǰ�Ļ��������H2��Cl2�����ʵ���֮��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ͨ�����㣬�������������±���
| H2��Cl2���ʵ����Ĺ�ϵ | ����NaCl�����ʵ��� |
�� | n(H2)=n(Cl2) |
|
�� | n(H2)��n(Cl2) |
|
(2)�ƶϵ�n��H2����n(Cl2)ʱ������NaCl�����ʵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��H2��Cl2��ɵĻ�����壬�����ճ�ַ�Ӧ��ͨ�뵽100 mL 1 mol��L-1��NaOH��Һ�У�ͼA��ʾ��Һ��ij�����ӵ����ʵ�������ͨ����������ı仯���仯�����ߣ�ͼB��ʾ��Һ�еĵ���������ͨ����������ı仯���仯�����ߡ�

(1)ͼA��ʾ��Һ��_____________________________���ӵı仯���ߣ�˵�������½���ԭ��____________________________________________________________________________��
(2)����Һ���е�����ʵ�飬��ͨ��Ļ�������������V1ʱ��������Һ������������ǿ����Ҫ��������___________��
(3)��n=0.02 molʱ������ǰ�Ļ��������H2��Cl2�����ʵ���֮��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ƹ���ѧ2010�����6����Ӧ�Կ��������������Ի�ѧA�� ���ͣ�ѡ����
��H2��Cl2��ɵĻ�����徭���ճ�ַ�Ӧ��ͨ��1000mL0.1 mol/L��NaOH��Һ��,ͼ�ױ�ʾij�����ӵ����ʵ�����ͨ�����������仯���ߡ���n=0.02molʱ������ǰ�Ļ��������H2��Cl2�����ʵ�����֮��Ϊ �� ��
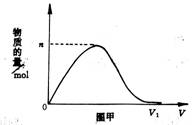
A��4��3 B��7��3
C��3��5 D��3��7
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com