
��10�֣�������Ԫ��A��B��C��D��E��Fԭ��������������A��C���γ�A2C2��A2C���ֻ����B������������Ӧ��ˮ�����������BA3�����������ӻ������ң�D��Aλ��ͬһ���壻E��C�γɵĻ������Ǵ�����Ⱦ������γ����ꣻFԪ������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ6��
��1��E��Ԫ�����ڱ��е�λ���� ��
��2����A��C��F����Ԫ�ذ�ԭ�Ӹ�����1��1��1��ɵĻ�������BA3��Ӧ����B�ĵ��ʣ� д���÷�Ӧ�Ļ�ѧ��ʽ ��
��3�������£����ס�������Һ��pH������5������ˮ������� = ������Һ���������ӵ����ʵ���Ũ���ɴ�С��˳���� ��
= ������Һ���������ӵ����ʵ���Ũ���ɴ�С��˳���� ��
��4����ͼ��ʾװ�õ����D��F�γ��εı�����Һʱ���������������������缫���������ĵ缫��ӦʽΪ ����Һ�г��ֵ������� ��
��1���������ڵڢ�A�壨2��3HClO+2NH3=3HCl+N2+3H2O��3��10-4��c��NO3-����c��NH4+����c��H+����c��OH-����4��Fe-2e-=Fe2+���������ݣ��а�ɫ������
�������������������Ԫ��A��B��C��D��E��Fԭ��������������E��C�γɵĻ������Ǵ�����Ⱦ������γ����꣬EΪ��Ԫ�ء�CΪ��Ԫ�أ�FԪ������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ6��F���ڢ�A�壬��FΪClԪ�أ�A��C���γ�A2C2��A2C���ֻ����A���ڢ�A�壬D��Aλ��ͬһ���壬D��ԭ������������Ԫ�أ���AΪԪ�ء�DΪNaԪ�ء�A2C2ΪH2O2��A2CΪH2O��B������������Ӧ��ˮ�����������BH3�����������ӻ������ң�BΪ��Ԫ�ء���Ϊ���ᡢ��Ϊ����李�BH3ΪNH3����1��EΪ��Ԫ�أ�ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ6���������ڱ��е������ڵڢ�A�壻
��2����H��O��Cl����Ԫ�ذ�ԭ�Ӹ�����1��1��1��ɵĻ�����ΪHClO����NH3��Ӧ���ɵ��������ݵ����غ��غ���HCl���ɣ�ͬʱ������ˮ����Ӧ����ʽΪ3HClO+2NH3=3HCl+N2+3H2O����3�������£�������Һ��ˮ����������ӵ�����Һ����������Ũ��Ϊ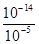 mol/L=10-9mol/L���������Һ��������Ϊˮ���������Ũ��Ϊ10-5mol/L������ˮ�������
mol/L=10-9mol/L���������Һ��������Ϊˮ���������Ũ��Ϊ10-5mol/L������ˮ�������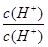 =
=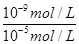 =10-4���������Һ�У�笠�����ˮ�⣬��Һ�����ԣ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳����c��NO3-����c��NH4+����c��H+����c��OH-������4������ͼ��ʾװ�õ����NaCl�ı�����Һʱ���������������������缫��������Fe�ŵ磬�����������ӣ������ĵ缫��ӦʽΪ Fe-2e-=Fe2+�����������ӷ���������������Һ�������������������ɣ����������ӷ�Ӧ��������������������������Ϊ���������ݣ��а�ɫ�������ɣ�
=10-4���������Һ�У�笠�����ˮ�⣬��Һ�����ԣ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳����c��NO3-����c��NH4+����c��H+����c��OH-������4������ͼ��ʾװ�õ����NaCl�ı�����Һʱ���������������������缫��������Fe�ŵ磬�����������ӣ������ĵ缫��ӦʽΪ Fe-2e-=Fe2+�����������ӷ���������������Һ�������������������ɣ����������ӷ�Ӧ��������������������������Ϊ���������ݣ��а�ɫ�������ɣ�
���㣺Ԫ�����ڱ���Ԫ�������ɣ�ˮ�ĵ��룻���ԭ��


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ʵ���У�Ҫ��ʹ�Ȼ�����Һ�е�Al3+��ȫ���������������ѡ�������Լ��е�
A��ʯ��ˮ B������������Һ c������ D����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����14�֣���֪������A��B�����붡�����Ԫ�طֱ���ͬ�������������ж�����ͬһ��Ԫ�ء�A��B�ڳ����¾���Һ̬��D��F��Ϊ���嵥�ʣ���D�� F���Ũ��Һ�ڳ����¶�����ֶۻ�������ʱ�д�����������������ת�������ַ�Ӧ������������δ�������
��1��A�Ľṹʽ �� E��F�ķ�Ӧ�� ���Ӧ���ƣ���д��2�ָ÷�Ӧ�ڹ�ҵ�����е���;�� �� ��
��2������Ӧ�ٳ����ɼ��⣬�����ɱ���д������D���ϡ��Һ��Ӧ�����ӷ���ʽ��
��3����A������Ի������ɼף�D���Ũ��Һ���������¿����������ֻ������ҡ�����B��д�����л�ѧ����ʽ�ڣ� �ۣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��5�֣������£�����A��B��C�ֱ�Ϊ���塢����ɫ��������ɫ���壬�ں��������£����ǿ�����������̽��з�Ӧ����֪E��Һ����ɫ�ġ���ش�
��1��д��E���ʵĻ�ѧʽ_______________��
��2��д��G��H�Ļ�ѧ����ʽ_____________________________________________��
��3��д��B��F��D�����ӷ���ʽ___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��ͼ �ֱ�����йط�Ӧ�е�һ�����ʣ�����д���¿հס�
�ֱ�����йط�Ӧ�е�һ�����ʣ�����д���¿հס�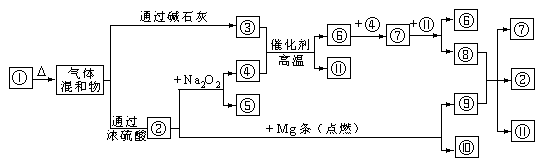
��1����֪�����������Ӹ�����Ϊ1:1����?�Ļ�ѧʽ�� ���ڵĵ���ʽΪ ��
��2����ͼ���漰��������ԭ��Ӧ���� ����
��3��?��?��Ӧ�Ļ�ѧ����ʽΪ ��
��4������ᷴӦ�Ļ�ѧ����ʽ�� ��
��5��һ�������£���2amL?��?�Ļ����������ˮ���ռ����õ�amL���壬��ԭ���������?��?�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ�������ȷ�Ӧ����
| A��̿ȼ������һ����̼ | B������кͷ�Ӧ |
| C��п����ϡH2SO4��Ӧ��ȡH2 | D��Ba(OH)2��8H2O��NH4Cl��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵���У�����ȷ����
| A���κλ�ѧ��Ӧ�е������仯������Ϊ�����仯 |
| B����ʹû�з�����ѧ�仯��Ҳ�����������ı仯 |
| C����ѧ��Ӧ�м������ʱ仯���������仯 |
| D�����ʵĻ�ѧ�ܿ���ͨ����ͬ�ı仯��ʽת��Ϊ���ܡ����ܵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ�����������������ڷ�Ӧ������������
| A��̼������ȷֽ� | B���Ҵ�ȼ�� |
| C����������������ĩ��Ӧ | D������������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����һ��ȡ����Ӧ��ֻ���������ַе㲻ͬ�IJ����������
| A��(CH3)2CHCH2CH2CH3 | B��(CH3CH2)2CHCH3 |
| C��(CH3)2CHCH(CH3)2 | D��(CH3)3CCH2CH3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com