
����ͼ��ʾ��װ���У���ֱͨ����5 minʱ��ͭ�缫��������2.16 g����ش��������⣺

(1)��Դ��X�缫Ϊֱ����Դ��________����
(2)pH�仯��A________(���������С�����䡱����ͬ)��B________��C________��
(3)ͨ��5 minʱ��B�й��ռ�224 mL(��״��)���壬��Һ���Ϊ200 mL����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
(4)��A��KCl��������Һ�����Ҳ��200 mL��������Һ��pHΪ________(����ǰ����Һ����ޱ仯)��
������ (1)����װ���Ǵ����ĵ��ء����AgNO3��Һʱ��Ag��������������ԭ��Ӧ������Ag�������������ӵ�ͭ�缫�������������缫��������Y��������X�Ǹ�����(2)A�ص��KCl��Һ����KOH����ҺpH����B�ص��CuSO4��Һ����H2SO4����ҺpH��С��C��Ϊ���������ҺpH���䡣(3)ͨ��5 minʱ��C������0.02 mol Ag����·��ͨ��0.02 mol���ӡ�B�й��ռ�0.01 mol���壬��������ȫΪ���������·����ͨ��0.04 mol���ӣ�����ת�Ʋ��غ㡣��ˣ�B�е���Ϊ�����Σ��ȵ��CuSO4��Һ����O2������ˮ����O2��H2��B���ռ�����������O2��H2�Ļ�������CuSO4��Һʱ����O2�����ʵ���Ϊx�����H2Oʱ����O2�����ʵ���Ϊy����4x��4y��0.02 mol(����ת���غ�)��x��3y��0.01 mol(�������֮��)�����x��y��0.002 5 mol������n(CuSO4)��2��0.002 5 mol��0.005 mol��c(CuSO4)��0.005 mol��0.2 L��0.025 mol/L��(4)ͨ��5 minʱ��A�зų�0.01 mol H2����Һ������0.02 mol KOH��c(OH��)��0.02 mol��0.2 L��0.1 mol/L��pH��13��
�𰸣�(1)����(2)����С������
(3)0.025 mol/L��(4)13



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������NaOH��������250mL1.25mol/L��NaOH��Һ����ղ���ش��������⣺
��1������250mL1.25mol/L��NaOH��Һ
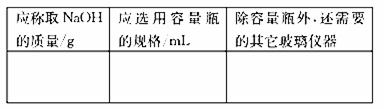 |
��2������ƿ����������������е�
���¶� ��Ũ�� ������ ��ѹǿ �ݿ̶���
��3������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������ƽȷ��ȡ�����NaOH����������������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C��������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1-2cm��
��4������A�У���ϴ��Һ����������ƿ����Ŀ���� ����Һע������ƿǰ��ָ������£�������Ϊ
��5���������Ƶ���ҺŨ��ƫС����
A��������ƿ��ת����Һʱ��ʵ�鲽��ڣ�������Һ����������ƿ����
B��������ˮʱ���������˿̶��ߡ�
C������ʱ���ӿ̶���
D������ǰ������ƿ������������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڸ�����������Һ�м������¸������ӣ�����������ԭ��Һ�д����������(����)
A���μӼ����Ժ�ɫ����Һ��Fe3����NH ��Cl����SCN��
��Cl����SCN��
B��pHֵΪ1����Һ��Cu2����Na����Mg2����NO
C��ˮ���������c(H��)��10��13 mol/L����Һ��K����HCO ��Br����Ba2��
��Br����Ba2��
D����������ΪNa2SO4����Һ��K����CO ��NO
��NO ��Pb2��
��Pb2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���е�Ũ�ȵ��������ʵ���Һ����CH3COOH����HClO����NaClO����H2CO3����Na2CO3����NaHCO3������ҺpH��С����������ȷ����(����)
A���ܢ٢ڢݢޢۡ��������� B���ܢ٢ڢޢݢ�
C���٢ܢڢޢۢ� D���٢ܢڢۢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�����в�����������ʵ��������(����)
A��������ˮϴ����ʽ�ζ��ܺ�װ���������еζ�
B��������ˮϴ����ƿ������NaOH��Һ��ϴ����װ��NaOH��Һ���еζ�
C���ü�ʽ�ζ���ȡ10.00 mL NaOH��Һ����������ˮϴ������ƿ�У���������������ˮ�ٽ��еζ�
D���÷�̪��ָʾ��������ɫ�ձ���ɫʱ��ֹͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ4NH3��g��+5O2(g) 4NO(g)+6H2O(g)��10 L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45 mol����˷�Ӧ��ƽ������v(X)����Ӧ����������ʻ���������������ʣ��ɱ�ʾΪ�� ��
4NO(g)+6H2O(g)��10 L�ܱ������н��У�����Ӻ�ˮ���������ʵ���������0.45 mol����˷�Ӧ��ƽ������v(X)����Ӧ����������ʻ���������������ʣ��ɱ�ʾΪ�� ��
A.v(NH3)=0.010 mol��(L��s)-1 B.v(O2)=0.001 mol��(L��s)-1
C.v(NO)=0.001 0 mol��(L��s)-1 D.v(H2O)=0.045 mol��(L��s)-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��25 ��ʱ����100 mL���Ȼ���14.6 g��������Һ�����5.6 g�����ۣ������Ƿ�Ӧǰ����Һ����ı仯������Ӧ��ʼ��2 minĩ�ռ�������1.12 L���ѻ���Ϊ��״�������ڴ�֮���־���4 min��������ȫ�ܽ⡣��
��1����ǰ2 min����FeCl2��ʾ��ƽ����Ӧ���ʣ�
��2���ں�4 min����HCl��ʾ��ƽ����Ӧ���ʣ�
��3��ǰ2 min���4 min��ȣ���Ӧ�����ĸ��Ͽ죿
�鿴�𰸺ͽ���>>
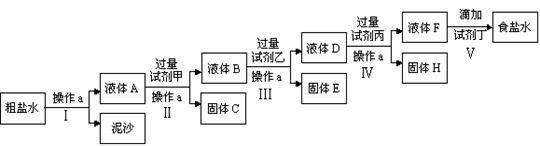
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ������������Ҫ�ɷ���Al2O3��Fe2O3��SiO2���ᴿAl2O3��ұ������ԭ�ϣ���ȡ�IJ������̿�����������ͼ��ʾ��[��֪��2NaAlO2+CO2+3H2O��Na2CO3+2Al(OH)3����SiO2�ܱ�NaOH�ܽ�]

��1����д�����и�����Ӧ�Ļ�ѧ����ʽ��
��________��________�� ��________��________��
��________��________�� ��________��________��
��2�����ٲ�û�й��˲�����ֱ�ӽ��벽��ڣ��Ժ���IJ�������ʲôӰ�죿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������ܱ������м���2 mol A��0.6 mol C��һ������B�������塣һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ(��)��ʾ������t0��t1��c(B)δ������ͼ(��)Ϊt2ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ��¶ȡ�ѹǿ�������е�һ������������t3��t4��Ϊʹ�ô�����
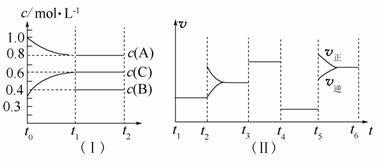
��ش��������⣺
(1)��t1��15 min����t0��t1����C���ʵ�Ũ�ȱ仯��ʾ��Ӧ����Ϊ____________________________��
(2)t4��t5�θı������Ϊ__________��B����ʼ���ʵ���Ũ��Ϊ__________������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
| t1��t2 | t2��t3 | t3��t4 | t4��t5 | t5��t6 |
| K1 | K2 | K3 | K4 | K5 |
��K1��__________(������λС��)��K1��K2��K3��K4��K5֮��Ĺ�ϵΪ________________________(�á���������������������)��
(3)t5��t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01 mol�����˹����������������Ƚ�������Ϊa kJ��д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ__________________________________��
(4)����ͬ�����£�����ʼʱ�����м���a molA��b mol B��c mol C��Ҫ�ﵽt1ʱ��ͬ����ƽ�⣬a��b��cҪ���������Ϊ__________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com