
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ú�ʵ�鷽�����г�������ʡ�ԣ�����ݴ���ش���Ӧ���⡣
�� ̽��Ũ�����ijЩ����
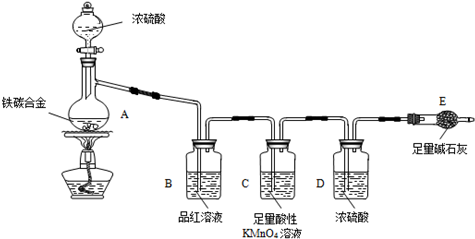
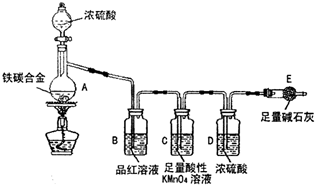
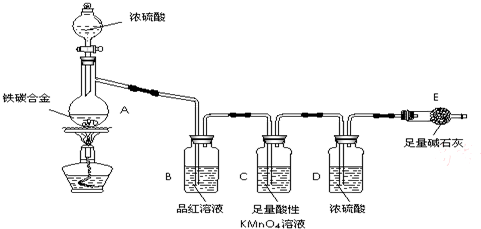
��1����ͼʾ����װ�ã����װ�õ������ԣ�����E��������
��2����a g��̼�Ͻ���Ʒ����A�У��ټ���������Ũ���ᡣ����A������Ϊ____________��
δ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�____ ___��
��3����ȼ�ƾ���һ��ʱ���A��B�пɹ۲쵽��������
A�п�ʼ������Ӧ�Ļ�ѧ����ʽΪ��2Fe +6H2SO4 Fe2��SO4��3 + 3SO2�� +6H2O
��______________________________________��д��ѧ����ʽ����
B�е�������_________,�ɴ˿ɵõ�Ũ�������_______�ԣ�Cװ�õ����� ��
��4�����ŷ�Ӧ�Ľ��У�A�л����ܷ���ijЩ���ӷ�Ӧ��д����Ӧ�����ӷ���ʽ__ __��
��5����Ӧһ��ʱ���A���ݳ������������Ȼ�Ͽ죬�����¶Ƚϸߣ���Ӧ�����⣬�����ܵ�ԭ����_____________________________________��
�� �ⶨ������������
��6����A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����ء�E����b g��
��̼�Ͻ���������������Ϊ_____________________(д����ʽ)��Ϊʹʵ�����ݸ�Ϊ��ȷ������װ�м�ʯ�ҵĸ���ܺ����________________________________��
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 11m-3b |
| 11m |
| 11m-3b |
| 11m |
m-
| ||
| m |
m-
| ||
| m |
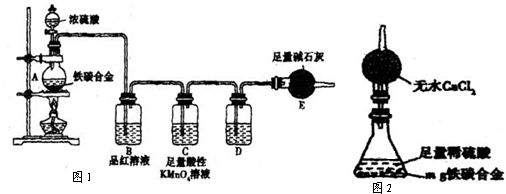
 ��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������
��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com