
��ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
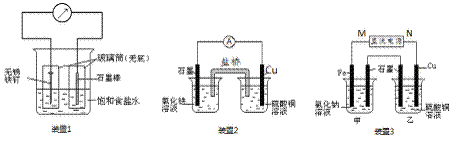
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0.64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0��2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0��5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0��64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0.64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�ϰ����ȹ���ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⡣
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ�����Һ����dz��ɫ���������� �����������ԭ�����������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���Ϊ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣����ձ��е��뼸�η�̪���۲쵽ʯī�缫�������ȱ�졣
�ٵ�Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�����ձ��е�ⷴӦ�����ӷ���ʽΪ ��
��ֹͣ��⣬ȡ��Cu�缫��ϴ�ӡ�����������缫����0.64 g�����ձ��в����������״�������Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�������Ĵ��¿���ѧ�Ծ� ���ͣ������
��12�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
 ��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0��2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0��5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0��64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com