
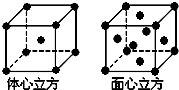
 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ�� ��
������ Bԭ�ӵ�p������������Χ�����Ų�ʽӦΪnS2nP3���γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ����ڰ�������֮�����������е����ͬ����������̬�⻯���BΪPԪ�أ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������ԭ�ӵĵ����Ų�ʽӦΪ1S22S22P63S23P5����DΪClԪ�أ�A+��Dԭ���γɵ�������һ�����Ӳ㣬��AΪNaԪ�أ�C��A�γ�A2C�����ӻ������CΪ�ڢ�A����Ԫ�أ����ݺ˵����A��B��C��D��E��֪��CΪSԪ�أ�E��ԭ������Ϊ26����ΪFeԪ�أ��ݴ˽��
��� �⣺Bԭ�ӵ�p������������Χ�����Ų�ʽӦΪnS2nP3���γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ����ڰ�������֮�����������е����ͬ����������̬�⻯���BΪPԪ�أ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������ԭ�ӵĵ����Ų�ʽӦΪ1S22S22P63S23P5����DΪClԪ�أ�A+��Dԭ���γɵ�������һ�����Ӳ㣬��AΪNaԪ�أ�C��A�γ�A2C�����ӻ������CΪ�ڢ�A����Ԫ�أ����ݺ˵����A��B��C��D��E��֪��CΪSԪ�أ�E��ԭ������Ϊ26����ΪFeԪ�أ�
��1��ͬһ����Ԫ�صĵ�һ�����ܴ����ҳ��������ƣ�PԪ��ԭ��3p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܵ�˳��Ϊ��Na��S��P��Cl���ʴ�Ϊ��Na��S��P��Cl��
��2��Fe2+�ĺ�������Ų�Ϊ1s22s22p63s23p63d6���۲�����Ų�ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��C���⻯��ΪH2S���������幹�ͺ�ˮ�������ƣ�ΪV�Σ�����������������IJ��ص���Ϊ���Է��ӣ��ʴ�Ϊ�����ԣ�
��3��PCl3�к���3���ļ����µ��Ӷ���Ϊ$\frac{5-1��3}{2}$=1�����Է��ӵĿռ乹����ƽ�����Σ��ʴ�Ϊ�������Σ�
��4��Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�۷е�ܵͣ������ڷǼ����ܼ���ӦΪ���Ӿ��������е����ʣ�Fe��CO��5��Fe��CO֮���γ���λ����Fe��CO��5�TFe��s��+5CO��Ӧ�����У����ѵĻ�ѧ��ֻ����λ�������ڷ�Ӧ����Fe�����γɽ�������
�ʴ�Ϊ�����Ӿ��壻��������
��5��������������ʵ�ʺ��е�Eԭ�Ӹ���Ϊ1+8��$\frac{1}{8}$=2����������������ʵ�ʺ��е�Eԭ�Ӹ���Ϊ6��$\frac{1}{2}$+8��$\frac{1}{8}$=4���ʶ��ߺ���ԭ����Ŀ֮��Ϊ2��4=1��2��
�ʴ�Ϊ��1��2��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų������Ӽ��ԡ��ռ乹�͡��������������ʡ���ѧ������������ȣ��ƶ�Ԫ�ص������ǽ����Ĺؼ�����Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ͻ�ԭ�������ʵ���֮��Ϊ1��14 | |
| B�� | �ɸ÷�Ӧ��֪��ԭ�ԣ�HCl��CrCl3 | |
| C�� | ��ת��0.2mol����ʱ���������Ļ�ԭ�������ʵ���Ϊ0.2mol | |
| D�� | Cl2�Ǹ÷�Ӧ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
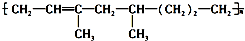 �����й�����������ȷ���ǣ�������
�����й�����������ȷ���ǣ�������| A�� | �ø߾�����ͨ���Ӿ۷�Ӧ���ɵ� | |
| B�� | �ϳɸø߾���ĵ��������� | |
| C�� | 1mol����������1molH2�ӳɣ����ɲ���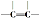 ������ ������ | |
| D�� | �ø߾����ܱ�����KMnO4��Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| A | B | C | |
| D |
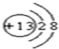
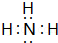 ��Y�ĽṹʽΪ
��Y�ĽṹʽΪ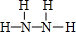 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.00mol NaCl���NA��NaCl���� | |
| B�� | 1.00mol NaCl�У�����Na+��������������Ϊ8NA | |
| C�� | ������1.00L��1.00mol•L-1��NaCl��Һ���ɽ�58.5g NaCl����1.00Lˮ�� | |
| D�� | ��״���£�11.2LCH3CH2OH�к��з��ӵ���ĿΪ0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
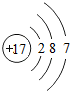 ��
�� ���û������ˮ��Һ�ڿ����о��ú���ʣ�����һ�ֵ��ʺ�һ��ǿ��û�ѧ����ʽ��ʾ��һ�仯���̲��õ����ű�ǵ���ת�Ƶķ������Ŀ��
���û������ˮ��Һ�ڿ����о��ú���ʣ�����һ�ֵ��ʺ�һ��ǿ��û�ѧ����ʽ��ʾ��һ�仯���̲��õ����ű�ǵ���ת�Ƶķ������Ŀ��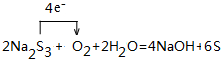
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ը�������Һ���� | B�� | ��NaOH��Һ����� | ||
| C�� | ������Cu��OH��2����Һ����� | D�� | ����ˮ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com