
(8��)��һ�ݻ��̶����ܱ������У�����һ�������������·�Ӧ��X��g��+2Y��g��3Z��g������֪�������м���2molX��3molY�ﵽƽ�������amolZ��
(1)����ͬʵ�������£�����ͬһ�����и�Ϊ����4molX��6molY���ﵽƽ���z�����ʵ���Ϊ ��
��2������ͬʵ�������£�����ͬһ�����и�Ϊ����3molX��2molY����Ҫ��ƽ��ʱ���������Z������������䣬�������Z mol��
��3������ͬ��ʵ�������£�����ͬһ�����и�Ϊ����0.5molX������� molY�� molZ������ʹƽ��ʱZΪ0.6amol��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����8�֣���һ�̶��ݻ����ܱ������У�����һ���¶ȣ���һ�������½������·�Ӧ��A(g)+2B(g)![]() 3C(g)����֪����1molA��3molB�Ҵﵽƽ���������a molC����
3C(g)����֪����1molA��3molB�Ҵﵽƽ���������a molC����
��1���ﵽƽ��ʱ��C�ڷ�Ӧ������е���������� ���ú���ĸa�Ĵ���ʽ��ʾ����
��2������ͬʵ�������£�����ͬһ�����и�Ϊ����2molA��6 mol B���ﵽƽ���C�����ʵ���Ϊ mol���ú���ĸa�Ĵ���ʽ��ʾ������ʱC�ڷ�Ӧ������е����������ԭƽ����� ��ѡ�������С�����䡱����
��3������ͬʵ�������£�����ͬһ�����и�Ϊ����2 mol A��8 mol B����Ҫ��ƽ���C�ڷ�Ӧ������������������ԭƽ����ͬ����Ӧ����C mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��) �Ӵ��������Ṥ���У�������Ӧ��450�沢�д��������½��У�
2SO2(g)+O2(g) 2SO3(g) ��H=��190 kJ��mo1��1
��1����һ�̶������г���2mol SO2��1molO2 ����һ���������´ﵽƽ�⣬��Ӧ�ų�������__________(����ڡ�С�ڻ����) 190 kJ
��2����һ���̶��ݻ�Ϊ5L���ܱ������г���0.20mol SO2��0.10molO2������Ӻ�ﵽƽ�⣬��������к�SO30.18mol����v(O2)=______mol��L-1��min-1
��3�����������ĸı���䷴Ӧ���ʼ�����Ӱ����� (ѡ�����)
�������¶� �ڱ���������䣬ֻ�������������� �۱���������䣬����Neʹ��ϵѹǿ���� �ܱ���ѹǿ���䣬����Neʹ�������������
��4��������������˵������(1)��Ӧ�Ѵ�ƽ����� (ѡ�����)
����v(O2)����2v(SO3)������SO2��O2 ��SO3��Ũ��֮��Ϊ2��1��2
�۵�λʱ��������2n molSO2��ͬʱ����2n mol SO3
�������������ƽ������������ʱ����仯
������������������ܶȲ���ʱ����仯 ������������ѹǿ����ʱ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ��������������Բ��Ի�ѧ�Ծ� ���ͣ������
(18��)1.��֪һ����ξ�Ϊ�����Ե�ǿ����ʡ���ij��Һ�пɷ������з�Ӧ��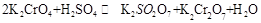
(1)�Խ�������Ӧ��д���������ʽ
��2�������ӷ�Ӧ��ƽ�ⳣ������ʽΪ��K= ��
��3��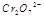 ��ˮ��Һ��Ϊ��ɫ��
��ˮ��Һ��Ϊ��ɫ��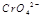 ��ˮ��Һ��Ϊ��ɫ��ij�����¸÷�Ӧ����ƽ�����ϵΪ�������ӵĻ��Һ����ɫΪ��ɫ��
��ˮ��Һ��Ϊ��ɫ��ij�����¸÷�Ӧ����ƽ�����ϵΪ�������ӵĻ��Һ����ɫΪ��ɫ��
����ˮ����ϡ�ͣ���Һ��ɫ��ƫ ���졢�ƣ�ԭ����
��
����������ƽ����ϵ����Һ���м�������ع�����ϵ��ɫ�кα仯��Ϊʲô��
��.�̶�������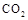 ,����Ч��������Դ�������ٿ����е��������塣��ҵ�������о�����
,����Ч��������Դ�������ٿ����е��������塣��ҵ�������о�����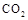 �������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
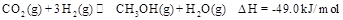
ij��ѧʵ�齫6mol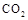 ��8molH
��8molH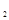 ����һ�ݻ�Ϊ2L���ܱ������У����H
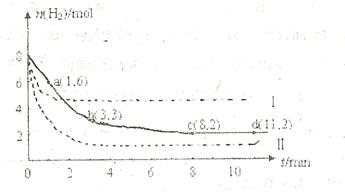
����һ�ݻ�Ϊ2L���ܱ������У����H �����ʵ�����ʱ��仯����ͼ��ʵ����ʾ��ͼ����ĸ�����ֱ�ʾ��Ӧ�����꣩��
�����ʵ�����ʱ��仯����ͼ��ʵ����ʾ��ͼ����ĸ�����ֱ�ʾ��Ӧ�����꣩��
���⣺
��1����ͼ������������ʱ����ڷ�Ӧ��������ʱ����� ����𰸱�ţ���
a.0��1min b.1��3min c.3��8min d.8��11min
(2)���ı�ijһ�����ٽ���ʵ�飬���H �����ʵ�����ʱ��仯��ͼ��������ʾ����ʵ����ȣ����ߢ�ı������������
�����ߢ�ı������������
�����ʵ�����ʱ��仯��ͼ��������ʾ����ʵ����ȣ����ߢ�ı������������
�����ߢ�ı������������
��
��3�����б����ܱ�ʾ�÷�Ӧ�Ѵ�ƽ����� ����𰸱�ţ�
a.������ѹǿ���ٸı� b.������������ܶȲ��ٸı�
c.����������ƽ��Ħ���������ٸı� d.�����ڸ����ʵ����ʵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ�߶�����������⻯ѧ�Ծ� ���ͣ������
(8��)��һ�ݻ��̶����ܱ������У�����һ�������������·�Ӧ��X��g��+2Y��g�� 3Z��g������֪�������м���2molX��3molY�ﵽƽ�������amolZ��
3Z��g������֪�������м���2molX��3molY�ﵽƽ�������amolZ��
(1)����ͬʵ�������£�����ͬһ�����и�Ϊ����4molX��6molY���ﵽƽ���z�����ʵ���Ϊ ��
��2������ͬʵ�������£�����ͬһ�����и�Ϊ����3molX��2molY����Ҫ��ƽ��ʱ���������Z������������䣬�������Z mol��
��3������ͬ��ʵ�������£�����ͬһ�����и�Ϊ����0.5molX������� molY�� molZ������ʹƽ��ʱZΪ0.6amol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com