
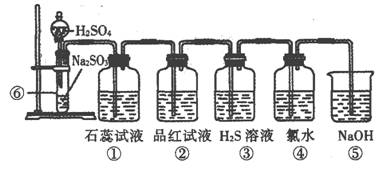
[s1] ��ͼ��ʵ������SO2����֤SO2ijЩ���ʵ�װ��ͼ��
�Իش�
(1)���з����Ļ�ѧ��Ӧ����ʽΪ��____________________________________��
(2)����ʵ������Ϊ________________��֤��SO2��ˮ��Ӧ����Һ��_____________��
(3)����Ʒ����Һ����Ϊ______________________��֤��SO2��____________�ԡ�
(4)����������_____________________��֤��SO2��_________�ԡ�
��Ӧ�Ļ�ѧ����ʽΪ ��
(5)����������_____________________��֤��SO2��_________�ԡ�
��Ӧ�����ӷ���ʽΪ ��
(6)�ݵ�������_____________________����Ӧ�����ӷ���ʽΪ��____________________��
[s1]28��
 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
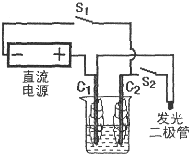 ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��
ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[s1] ��ͼ��ʵ������SO2����֤SO2ijЩ���ʵ�װ��ͼ��

�Իش�
(1)���з����Ļ�ѧ��Ӧ����ʽΪ��____________________________________��
(2)����ʵ������Ϊ________________��֤��SO2��ˮ��Ӧ����Һ��_____________��
(3)����Ʒ����Һ����Ϊ______________________��֤��SO2��____________�ԡ�
(4)����������_____________________��֤��SO2��_________�ԡ�
��Ӧ�Ļ�ѧ����ʽΪ ��
(5)����������_____________________��֤��SO2��_________�ԡ�
��Ӧ�����ӷ���ʽΪ ��
(6)�ݵ�������_____________________����Ӧ�����ӷ���ʽΪ��____________________��
[s1]28��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�á�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5![]() Na2SO4��Һ����Դ��3~6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��
Na2SO4��Һ����Դ��3~6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��

��1����̼���缫�����������������__________
��2�����¿���S1������Ӻ���̼���ϲ������Ե����ݣ���ʱ̼��C1Ϊ__________����װ���з������ܷ�Ӧ�Ļ�ѧ����ʽΪ__________��
��3���Ͽ�����S1�����¿���S2���ɹ۲쵽����������Ϊ__________��̼��C2Ϊ__________������缫��ӦʽΪ__________��ks5u
��4����С���0.5![]() Na2SO4��Һ����0.5
Na2SO4��Һ����0.5![]() NaCl��Һ�����Ͽ�����S2�����¿���S1�������C1�����������ʵ�鷽����__________��һ��ʱ����Ͽ�����S1�����¿���S2ʱ���缫C1��ӦʽΪ__________��
NaCl��Һ�����Ͽ�����S2�����¿���S1�������C1�����������ʵ�鷽����__________��һ��ʱ����Ͽ�����S1�����¿���S2ʱ���缫C1��ӦʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com