
���� ��1������M��Z�����ԭ������֮��Ϊ3��5������M��Z�����ԭ�������ֱ�Ϊ3x��5x��
����MCO3•ZCO3��MCO3��ZCO3�ı�ֵΪ1��1���ʵõ���������MO��ZO�����ʵ���֮��ҲΪ1��1������MCO3•ZCO3������Ϊ1.84g���õ������������Ϊ0.96g���ɵã�
$\frac{3x+5x+32}{3x+5x+120}$=$\frac{0.96}{1.84}$�����ɽ��xֵ���Ӷ��ó�M��Z�����ԭ�����������ó���ʯ�Ļ�ѧʽ��
��2�����������պ�IJ���ΪCaO��MgO�Ļ�������ո����������õ��ʹ軹ԭ�����õ�����Mg��һ�ֺ������Σ��ݴ�д����ѧ����ʽ���ڵ�����ڵ��Ȼ�þ�õ�����ˮ��Һ�е�⣬þ���Ӳ����������õ�������þ���ò�������þ��
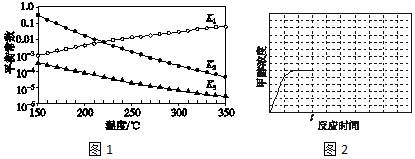
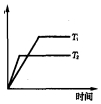
��3����Ӧ1��2��3���Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2��K3������ͼ1�������¶ȱ仯�����߽�ϸ�˹���ɽ��з������
��4�����¶�T1ʱ��ʹ�����Ϊ3��1��H2��CO2������㶨���ܱ������ڽ��з�ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����H3��0��tʱ��Ѹ�ٽ��µ�T2�������¶�ƽ��������Ӧ�����ƶ���CH3OHŨ�����ݴ˻���tʱ�̺�״�Ũ����ʱ��仯��ƽ���ʾ�����ߣ�
��� �⣺��1������M��Z�����ԭ������֮��Ϊ3��5������M��Z�����ԭ�������ֱ�Ϊ3x��5x��
����MCO3•ZCO3��MCO3��ZCO3�����ʵ���֮��Ϊ1��1���ʵõ�����������MO��ZO�����ʵ���֮��ҲΪ1��1������MCO3•ZCO3������Ϊ1.84g���õ������������Ϊ0.96g���ɵã�
$\frac{3x+5x+32}{3x+5x+120}$=$\frac{0.96}{1.84}$��x=8��M�����ԭ������Ϊ3x=24����MΪMg��Z�����ԭ������Ϊ5x=40����ZΪCa�����ʯ�Ļ�ѧʽΪMgCO3•CaCO3��
�ʴ�Ϊ��MgCO3•CaCO3��
��2�����������պ�IJ���ΪCaO��MgO�Ļ�������ո����������õ��ʹ軹ԭ�����õ�����Mg��һ�ֺ������Σ����ڴ˺���������ֻ��Z��Si��OԪ�أ���Z��Si�����ʵ���֮��Ϊ2��1����ΪCa2SiO4���ʴ˷�Ӧ�Ļ�ѧ����ʽΪ��2MgO+2CaO+Si$\frac{\underline{\;��ո���\;}}{\;}$2Mg+Ca2SiO4��
�ʴ�Ϊ��2MgO+2CaO+Si$\frac{\underline{\;��ո���\;}}{\;}$2Mg+Ca2SiO4��
����Һ�к��е������ӵķŵ�˳��Ϊ��H+��Mg2+�������ӵķŵ�˳��Ϊ��Cl-��OH-�����MgCl2��Һʱ��������H+��Mg2+���õ����ӣ��缫��ӦʽΪ2H2O+2e-=H2��+2OH-�����Եò���þ���ʣ�
�ʴ�Ϊ�����MgCl2��Һʱ��������H+��Mg2+���õ����ӣ��缫��ӦʽΪ2H2O+2e-=H2��+2OH-�����Եò���þ���ʣ�
��3����ͼl��֪�������¶����ߣ�K1������Ӧ1��CO2��g��+H2��g��?CO��g��+H2O��g����H1��0����Ӧ3�ɷ�Ӧ1+2���ã����ݸ�˹���ɣ���H3=��H1+��H2�����ԡ�H2����H3��
�ʴ�Ϊ��С�ڣ���ͼl��֪�������¶����ߣ�K1�������H1��0�����ݸ�˹�����ֵá�H3=��H1+��H2�����ԡ�H2����H3��
��4����ͼl��֪�������¶����ߣ�K3��С����Ӧ3��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H3��0��tʱ��Ѹ�ٽ��µ�T2�������¶�ƽ��������Ӧ�����ƶ���CH3OHŨ������tʱ�̺�״�Ũ����ʱ��仯��ƽ���ʾ������Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���Ϊ�ۺϣ��漰���ӻ�����Ļ�ѧʽ��ȷ���ͻ�ѧ����ʽ����д�����ԭ�����缫��Ӧʽ����д����ѧƽ���֪ʶ��Ϊ�߿��������ͣ��������ո�˹���ɵ�Ӧ�á���Һ�����ӵķŵ�˳��ѧƽ���ƶ�ԭ����֪ʶ�ǽ������Ĺؼ�����Ŀ�ѶȽϴ���ѧ���ķ�����������������ѧ����������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
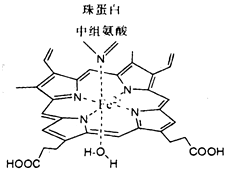
 �����£���1LpH=10��NaOH��Һ�г���ͨ��CO2��ͨ��CO2�������V������Һ��ˮ�������c��OH-���Ĺ�ϵ��ͼ��ʾ��������������ȷ���ǣ�������
�����£���1LpH=10��NaOH��Һ�г���ͨ��CO2��ͨ��CO2�������V������Һ��ˮ�������c��OH-���Ĺ�ϵ��ͼ��ʾ��������������ȷ���ǣ�������| A�� | a����Һ�У�ˮ�������c��H+��=1��10-10mol•L-1 | |
| B�� | b����Һ�У�c��H+��=1��10-mmol•L-1 | |
| C�� | c����Һ�У�c��Na+����c��HCO3-����c��CO32-�� | |
| D�� | d����Һ�У�c��Na+��+c��H+���T2c��CO32-��+c��HCO3-��+c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ϸ����Ѫ���ؽṹ��ͼ��ʾ���ش��������⣺
��ϸ����Ѫ���ؽṹ��ͼ��ʾ���ش��������⣺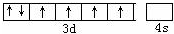 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Q1��Q2 | B�� | Q1��Q2 | C�� | Q1=Q2 | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ�� | ���� | ���� |
| A | ��FeCl3��Һ����Mg��OH��2����Һ�� | �۲쵽�����ɰ�ɫ��Ϊ���ɫ | Fe��OH��3���ܽ�ȴ���Mg��OH��2 |
| B | ��ij��Һ�еμ�BaCl2��Һ������ϡ�����ữ | ��Һ�в�����ɫ��������ϡ����ܽ� | ԭ��Һ��һ������SO42- |
| C | ��ϡ�����ữ��H2O2��Һ����Fe��NO3��2��Һ�� | ��Һ���ɫ | �����ԣ�H2O2��Fe3+ǿ |
| D | ���з�̪��Na2CO3��Һ�м�������BaCl2���� | ��Һ��ɫ��dz | ֤��Na2CO3��Һ�д���ˮ��ƽ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������89 | B�� | ��������������֮��Ϊ50 | ||
| C�� | �����������39 | D�� | ${\;}_{39}^{89}$Y��${\;}_{39}^{90}$Y��Ϊͬλ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ����FeCl3��Һ���Ƿ����FeCl2 | ����Һ�еμ�KSCN��Һ���ٵμ���ˮ |
| B | ֤��H2CO3���Ա�H2SiO3ǿ | Na2CO3��SiO2�ڸ��������ڷ�Ӧ |
| C | ��ȥCu���л��е�CuO | �������еμ�����ϡ���Ტ���� |
| D | ����δ֪��Һһ������CO32- | ����ϡ��������ɫ��ζ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOHֻ��NH4HSO4��Ӧ | |
| B�� | ��NH4��2SO4��ˮ�еĵ��뷽��ʽ����NH4��2SO4=NH4++SO42- | |
| C�� | NH4HSO4�����ʵ�����0.04 mol | |
| D�� | ��NH4��2SO4��NH4HSO4���ʵ���֮����1.87��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �о���������CuZnO2���������£�CO2��H2�ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ���£�
�о���������CuZnO2���������£�CO2��H2�ɷ�������ƽ�з�Ӧ���ֱ�����CH3OH��CO����Ӧ���Ȼ�ѧ����ʽ���£��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com