±¾ĢāĪŖŃ”×öĢā£¬°üĄØA”¢BĮ½Ģā£®Ń”ѧ”¶»ÆѧÓėÉś»ī”·Ä£æéµÄæ¼Éś“šAĢā£¬Ń”ѧ”¶ÓŠ»ś»Æѧ»ł“””·Ä£æéµÄæ¼Éś“šBĢā£¬ĆæĪ»æ¼ÉśÖ»æÉŃ”×ö1Ģā£®ČōĮ½Ģā¶¼×÷“š£¬ŌņŅŌAĢā¼Ę·Ö£®
A£®”¶»ÆѧÓėÉś»ī”·
£Ø1£©²ÄĮĻŹĒČĖĄąĄµŅŌÉś“ęŗĶ·¢Õ¹µÄÖŲŅŖĪļÖŹ»ł“”£®
¢ŁÉś²śĖ®ÄąµÄÖ÷ŅŖŌĮĻŹĒš¤ĶĮŗĶ
ŹÆ»ŅŹÆ
ŹÆ»ŅŹÆ
£ØĢīĆū³Ę£©£®
¢ŚÓŠ»ś²£Į§£Ø¾Ū¼×»ł±ūĻ©Ėį¼×õ„£©Źµ¼ŹÉĻ²»ŹĒ¹čĖįŃĪ²ÄĮĻ£¬¶ųŹĒŅ»ÖÖ
ĖÜĮĻ
ĖÜĮĻ
£®£ØŃ”Ģī”°Ļš½ŗ”±»ņ”°ĻĖĪ¬”±»ņ”°ĖÜĮĻ”±£©
¢ŪÉĻŗ£ŹĄ²©»įÖŠ¹ś¹ŻÓƵ½Ņ»ÖÖĶæĮĻ-·śĢ¼Ķæ²ć¾Ūõ„£ØFEP£©£¬ĖüµÄµ„ĢåĪŖCF
3-CF=CF
2£¬ÕāÖÖĶæĮĻ¾ßÓŠ
ČČĖÜŠŌ
ČČĖÜŠŌ
£ØŃ”Ģī”°ČČĖÜŠŌ”±»ņ”°ČČ¹ĢŠŌ”±£©£®
¢Ü²£Į§øÖ¾ßÓŠÄĶøÆŹ“”¢ÖŹĮæĒį”¢Ēæ¶ČøߵĊŌÄÜ£¬ĖüŹōÓŚ
ø“ŗĻ
ø“ŗĻ
²ÄĮĻ£ØŃ”Ģī”°ŗĻ½š”±»ņ”°ø“ŗĻ”±£©£®
£Ø2£©ČĖĢå½”æµÓėŹ³Ę·”¢Ņ©ĪļµČ¹ŲĻµĆÜĒŠ£®
¢ŁÓŠŠ©Ń§Éś“ÓŠ”Ę«Ź³£¬²»°®³ŌĖ®¹ū”¢Źß²Ė£¬½į¹ūÓŖŃųȱ·¦”¢·¢Óż²»Į¼£¬ÕāÖ÷ŅŖŹĒÓÉÓŚÉćČ”
Ī¬ÉśĖŲ
Ī¬ÉśĖŲ
£ØŃ”Ģī”°Ö¬·¾”±”¢”°Ī¬ÉśĖŲ”±»ņ”°µ°°×ÖŹ”±£©²»×ćŅżĘšµÄ£®
¢ŚÉś»īÖŠÓ¦ŗĻĄķµŲÉćČ”ČĖĢå±ŲŠčµÄŌŖĖŲ£¬ĢåÄŚ
µā
µā
ŗ¬Įæ¹żøߣ¬»įŅżĘš¼×דĻŁ¼²²”£®
¢ŪĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹ¶¼ŹĒČĖĢå±ŲŠčµÄÓŖŃųĪļÖŹ£®ĘäÖŠµ°°×ÖŹ±»ÉćČėČĖĢåŗó£¬ŌŚĆøµÄ×÷ÓĆĻĀĖ®½āĪŖ
°±»łĖį
°±»łĖį
£ØŠ“Ćū³Ę£©£®
¢ÜŠ”ĖÕ“ņæÉÓĆĄ“ÖĪĮĘĪøĖį¹ż¶ą£¬ĒėŠ“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½
HCO3-+H+=CO2ӟ+H2O
HCO3-+H+=CO2ӟ+H2O
£®
¢ŻæÉŅŌ°ļÖśĮ÷øŠ²”ČĖ½āČČÕņĶ“µÄŅ©ĪļŹĒ
°¢Ė¾Ę„ĮÖ
°¢Ė¾Ę„ĮÖ
£ØŃ”Ģī”°ĒąĆ¹ĖŲ”±»ņ”°°¢Ė¾Ę„ĮÖ”±£©£®
£Ø3£©½ą¾»°²Č«µÄÉś“ę»·¾³øüŹĒ½”浵ı£ÕĻ£®
¢ŁĪŖČ·±£ÉĻŗ£ŹĄ²©»įĘŚ¼ä³ĒŹŠæÕĘųÖŹĮæÓÅĮ¼ĀŹ“ļµ½95%ŅŌÉĻ£¬ŹĄ²©»įĘŚ¼äµÄæÕĘųÖŹĮæדæö¼ą²āÖŠ£¬²»ŠčŅŖ¼ą²āµÄÖø±źŹĒ
d
d
£®
a£®æÉĪüČėæÅĮ£Īļ£ØPM
10£© b£®NO
2ÅØ¶Č c£®SO
2ÅØ¶Č d£®CO
2ÅضČ
¢Ś×ŌĄ“Ė®æÉĄūÓĆĘÆ°×·ŪĄ“ɱ¾śĻū¶¾£¬ĘäÄÜɱ¾śĻū¶¾µÄŌŅņ£ØÓĆ»Æѧ·“Ó¦·½³ĢŹ½±ķŹ¾£©ŹĒ
Ca£ØClO£©2+CO2+H2O=CaCO3+2HClO
Ca£ØClO£©2+CO2+H2O=CaCO3+2HClO
£®
¢ŪĄ¬»ųµÄĪŽŗ¦»Æ“¦Ąķ³£ÓƵķ½·Ø°üĄØĪĄÉśĢīĀńŗĶ
·ā±ÕŹ½
·ā±ÕŹ½
·ŁÉÕ£®
¢ÜČĖĆĒĪŖ×·ĒóŹ±ÉŠ¶ų°ŃĶ··¢Č¾³Éø÷ÖÖø÷ŃłµÄŃÕÉ«£¬³£¼ūµÄČ¾·¢¼ĮÓŠÖ²ĪļČ¾·¢¼Į”¢ĪŽ»śČ¾·¢¼ĮŗĶ
ŗĻ³É
ŗĻ³É
Č¾·¢¼Į£ØŃ”Ģī”°ÓŠ»ś”±”°ŗĻ³É”±»ņ”°ø“ŗĻ”±£©£®
B£®£Ø1£©¾ßÓŠĆ÷ĻŌĻÖĻóµÄ¹ŁÄÜĶÅĢŲÕ÷·“Ó¦³£±»ÓĆĄ“¼ų¶Ø»ÆŗĻĪļ£®
¢ŁÓūĒų·ÖCH
2=CH
2ŗĶCH
3CH
3£¬Ó¦Ń”ÓĆ
b
b
£ØĢī×ÖÄø£©£®
a£®NaOHČÜŅŗ b£®äåĖ® c£®Ņų°±ČÜŅŗ
¢ŚÓūĒų·ÖHCHOŗĶHCOOH£¬Ó¦Ń”ÓĆ
c
c
£ØĢī×ÖÄø£©£®
a£®KMnO
4ČÜŅŗ b£®Ņų°±ČÜŅŗ c£®Na
2CO
3ČÜŅŗ
¢ŪÓūĒų·Ö
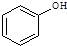
ŗĶ
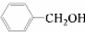
£¬Ó¦Ń”ÓĆ
a
a
£ØĢī×ÖÄø£©£®
a£®FeCl
3ČÜŅŗ b£®NaOHČÜŅŗ c£®AgNO
3ČÜŅŗ
£Ø2£©ŌŚÓŠ»ś»Æѧ֊£¬Ķ¬·ÖŅģ¹¹ŹĒĘÕ±é“ęŌŚĻÖĻó£®·Ö×ÓŹ½ĪŖC
4H
9OHµÄÓŠ»śĪļ¹²ÓŠ
4
4
ÖÖ£®ĘäÖŠ£¬Ņ»ÖÖÓŠ»śĪļĶعżĻūČ„·“Ó¦æÉ×Ŗ±äĪŖ2-¶”Ļ©£¬ĒėŠ“³öøĆĻūČ„·“Ó¦µÄ»Æѧ·½³ĢŹ½
H
3CCH
2CH£ØOH£©CH
3CH
3CH=CHCH
3+H
2O
H
3CCH
2CH£ØOH£©CH
3CH
3CH=CHCH
3+H
2O
£»ĮķŅ»ÖÖÓŠ»śĪļµÄŗĖ“Ź²ÕńĒāĘ×Ķ¼£Ø
1HŗĖ“Ź²ÕńĘ×Ķ¼£©ÖŠĻŌŹ¾Ņ»øö·å£¬ĒėŠ“³öøĆÓŠ»śĪļµÄ½į¹¹¼ņŹ½ČēĻĀ£ŗ
£®
£Ø3£©AŹĒŹÆÓĶĮŃ½āĘųµÄ³É·ÖÖ®Ņ»£¬AµÄijŅ»Ķ¬ĻµĪļEµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½£®ĻÖŅŌAĪŖÖ÷ŅŖŌĮĻŗĻ³ÉC
6H
12O
2£¬ĘäŗĻ³ÉĀ·ĻßČēĶ¼ĖłŹ¾£ŗ
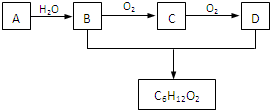
»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ
CH3CH=CH2
CH3CH=CH2
£®
¢ŚB”¢D·Ö×ÓÖŠµÄ¹ŁÄÜĶÅĆū³Ę·Ö±šĪŖ
ōĒ»ł
ōĒ»ł
Ӣ
ōČ»ł
ōČ»ł
£®
¢ŪŠ“³öBµÄĶ¬Ąą±šµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ
£®
¢ÜŠ“³öB”śCµÄ»Æѧ·½³ĢŹ½£ŗ
2CH
3CH
2CH
2OH+O
22CH
3CH
2CHO+2H
2O
2CH
3CH
2CH
2OH+O
22CH
3CH
2CHO+2H
2O
£®


 æŖŠÄæģĄÖ¼ŁĘŚ×÷ŅµŹī¼Ł×÷ŅµĪ÷°²³ö°ęÉēĻµĮŠ“š°ø
æŖŠÄæģĄÖ¼ŁĘŚ×÷ŅµŹī¼Ł×÷ŅµĪ÷°²³ö°ęÉēĻµĮŠ“š°ø ĆūĢāѵĮ·ĻµĮŠ“š°ø
ĆūĢāѵĮ·ĻµĮŠ“š°ø ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
 ŗĶ
ŗĶ £¬Ó¦Ń”ÓĆ
£¬Ó¦Ń”ÓĆ














